12884 0
During hysteroscopy in the first half of the proliferation phase (until the 7th day of the cycle), the endometrium is pale, thin, with small hemorrhages and single pale pink areas of non-rejected endometrium. Mouths fallopian tubes well distinguishable.
In the second half of the proliferation phase (from the 9th day of the cycle), the endometrium is pale Pink colour, thickened, vessels are not pronounced. Later, thickened longitudinal or transverse folds are distinguished in certain areas.
In the secretion phase, the endometrium yellowish color, thickened. Folds that are especially well expressed in the upper third of the body of the uterus are identified. 2-3 days before menstruation, the endometrium is red with areas of dark purple rejection. The openings of the fallopian tubes may be hidden by the folds of the endometrium.
The first 2-3 days during menstruation, the uterine cavity is filled with rejected layers of the endometrium: in the upper third it is dark purple in color, in the lower and middle third it is pale pink.
During the postmenopausal period, hysteroscopy reveals a picture of endometrial atrophy. In this case, the endometrium is thinned and has a pale color.
During colposcopy, the mucous membrane of the cervix is smooth, shiny, and pink.
In postmenopausal women, thinning of the epithelium is normally detected, through which the vessels are visible.
During laparoscopy, the unchanged uterus is covered with shiny peritoneum, has a smooth surface and a characteristic shape with symmetry relative to the longitudinal plane.
With hysterosalpingography, the shadow of the uterine cavity has the shape of a triangle with slightly concave sides and clear, even contours. The base of the triangle faces up and the apex faces down.
The upper corners correspond to the openings of the fallopian tubes, the lower corner corresponds to the internal opening of the cervical canal. The uterine cavity holds from 4 to 6 ml of contrast liquid.
With ultrasonography, the contours of a normal uterus are clear and even, oval or pear-shaped. The echo density of the endometrium is higher than the echo density of the myometrium, which does not change depending on the phase menstrual cycle. The echostructure of the unchanged myometrium is finely dispersed due to many point and linear echo signals.
The endometrium is defined as an echo-positive formation that is linear (after the end of menstrual bleeding), oval or drop-shaped. Immediately after the end of the menstrual cycle, the endometrium can be traced in the form of an echo-positive strip 1-2 mm thick.

On days 8-10 of the cycle (the middle of the proliferation phase), the endometrium thickens somewhat, on average up to 8 mm (from 5 to 10 mm). The echo structure remains virtually unchanged compared to the previous period.

In the late proliferation phase (11-14 days), in addition to further thickening, on average up to 11 mm (from 7 to 14 mm), the echogenicity of the endometrium begins to increase slightly and becomes close to average.

In the early secretion phase (days 15-18), the rate of endometrial growth decreases, it reaches a thickness of 12 mm. The echogenicity of the endometrium continues to increase from the periphery to the center, as a result of which the hypoechoic central fragment takes on a teardrop shape ( wide part in the area of the fundus of the uterus it narrows towards the cervix). During this phase, the hyperechoic line in the center is no longer clearly visible.

In the mid-secretion phase (days 19-23), the endometrium reaches its maximum thickness - an average of 14 mm (from 12 to 18 mm). The echogenicity of the endometrium increases even more; the hyperechoic line in the center is not clearly visualized.

On days 24-27 of the cycle (late secretion), the thickness of the endometrium decreases slightly - to an average of 12 mm (from 10 to 17 mm). A feature of this period is the high echogenicity of the endometrium in combination with its heterogeneous internal echostructure, due to which the closure line ceases to be visualized.

During menstruation, a thin hyperechoic stripe or hyperechoic echostructures (blood clots) are detected in the uterine cavity. Sometimes the cavity appears slightly dilated due to echo-negative contents.

The uterine cavity in postmenopause is an M-echo in the form of a thin hyperechoic line, usually 1-2 mm (no more than 4-5 mm) thick.

When nuclear magnetic tomography (NMT) in the first half of the cycle, the endometrium on the median sagittal section is determined as a thin line (up to 3 mm), the myometrium looks like a homogeneous structure with smooth contours.

In the second half of the cycle, the endometrium is visualized as a fairly homogeneous structure with an average thickness of 7 mm, more intense than the myometrium.

In the postmenopausal period, tomograms reveal a decrease in the volume of the uterus with a decrease in the intensity of the image of the myometrium, while the endometrium, as a rule, is not visualized.

The cervix is defined on tomograms as a non-intense cylindrical zone with a clear smooth outline, the structure and cavity of which correspond to the body of the uterus. Ultrasonography usually does not visualize the cervical canal.
V.N. Serov, I.N. Zvenigorodsky
Dueholm, C. Møller, S. Rydbjerg, E. S. Hansen, G. Ørtoft
P.G.Leone, D.Timmerman, T.Bourne, L.Valentin, E.Epstein, S.R.Goldstein, H.Marret, A.K.Parsons, B.Gull, O.Istre, W.Sepulveda, E.Ferrazzi, T.Van den Bosch
Transvaginal ultrasound examination It has great importance in the diagnosis of endometrial cancer in women with postmenopausal bleeding. Women with an endometrial thickness ≤ 4 mm measured by transvaginal scanning have a low risk of developing endometrial cancer (1 in 100 cases) if they do not take hormone replacement therapy. hormone therapy, 1 in 1000 if they are taking therapy). Women with postmenopausal bleeding and endometrial thickness ≥ 5 mm have high risk endometrial cancer (1 in 4 cases), so it is necessary to obtain a high-quality intrauterine scraping for histological analysis. Ultrasound can provide information about individual risk malignant neoplasms in postmenopausal women with bleeding and endometrial thickness ≥ 5 mm.
Our study included women with postmenopausal bleeding and endometrial thickness ≥ 5 mm, as measured by a transvaginal probe. The study was conducted at the University Hospital in Aarhus, Denmark, between November 2010 and February 2012. All women underwent transvaginal scanning (TVS) and gel infusion sonography (GIS), and all were scheduled for hysteroscopy with resectoscopic biopsy and additional curettage to evaluate the intrauterine pathologies (Table 1).
Table 1. Patient selection scheme for the study.
Transvaginal scan (TVS)
TVS was performed on a Voluson E8 Expert equipped with an endovaginal sensor (6-12 MHz), according to the scanning protocol. The Doppler parameters were set in advance, standardized (frequency 6 MHz, Doppler power gain 50, dynamic range 10 dB; persistence 2, map color 1, filter 3).
The TVS scan included a visual assessment of the following parameters, determined International Group Endometrial Tumor Analysis (IETA): endometrial thickness, its echogenicity (hyper-, hypo-, and isoechoic, homo/heterogeneous), cystic component (yes/no), if present, then smooth or uneven limits, endometrial boundaries (smooth or uneven , homo-/heterogeneous), closure line (yes/no), interrupted (yes/no)).
Power Doppler analysis included a visual assessment of the following parameters: vessels (present (yes / no), presence of a dominant vessel (yes / no), if there is a dominant vessel, then single (yes / no) or double (yes / no), origin (focal / multifocal) multiple vessels (yes / no); branching (yes / no), if there are branches, then ordered / disordered, circular direction of the vessels (yes / no). We assessed subjectively: large vessels (yes / no), color Doppler (yes/no), vascular density (yes/no).
Gel infusion sonography (GIS)
GIS was carried out after TVS. We used a small flexible sterile catheter equipped with a 10 ml syringe containing Instillagel® (E.Tjellesen A/S, Lynge, Denmark) which was inserted into the uterine cavity. In patients with an obstructed cervix, we used a small Hegar dilator. The gel was introduced into the uterine cavity under ultrasound control.
The uterine cavity was then scanned in the sagittal and transverse planes, assessing the same parameters as for conventional TVS. The following were also assessed: the presence of formation, its location and the percentage of endometrial damage (that is, ≤ 25% of the surface is damaged) (yes/no); Surface structure of local damage (uniform / uneven); The structure of the general surface of the endometrium (smooth, polypoid, uneven).
Hysteroscopy
Outpatient hysteroscopy was performed in all patients using local or general anesthesia. In 112 patients, hysteroscopy was performed immediately after the ultrasound examination, in other patients at the next visit within 3 weeks after the ultrasound examination. During hysteroscopy, attempts were made to remove all tissue from the uterine cavity. Three to five endometrial samples were collected from one patient.
Calculation of the risk of developing endometrial cancer using a scoring system
(Risk of endometrial cancer score (REC score))
Based on our analyses, we developed a risk scoring system for endometrial cancer (Fig. 1). The scoring system included body mass index (≥30 = 1 point), endometrial thickness (≥10mm = 1 point), (≥15mm = 1 point), presence of vascularization, dominant vessel (present = 1 point), multiple vessels (present = 1 point), large vessels (present = 1 point) and dense vessels (present = 1 point), discontinuous endomyotrial zone (present = 1 point) and uneven endometrial surface on GIS (present = 1 point). Adding these values creates an endometrial cancer risk score. Score 3 for TVS or 4 for GIS showed good scan results and correctly diagnosed high level development of endometrial cancer in about 90% of all patients.

Fig.1. Schematic representation of determining the risk of developing endometrial cancer using a scoring system.
Ultrasound examination parameters of the endometrium were determined by the International Endometrial Tumor Analysis Group (IETA)
Endometrial thickness is measured in the sagittal plane. Calipers should be placed at the endometrial-myometrial interface, perpendicular to the endometrial midline (Fig. 2). When fluid is present, then the thickness of individual parts of the endometrium is measured and their sum is recorded (Fig. 2b).

Fig.2. Schematic and ultrasound image of endometrial measurement in normal conditions (a), and in the presence of intrauterine fluid (b).
The echogenicity of the endometrium is assessed in comparison with the echogenicity of the myometrium as hyperechoic, isoechoic or hypoechoic.
The homogeneity of the endometrium is assessed by its structure. “Homogeneous” endometrium is homogeneous and has a three-layer structure (Fig. 3). “Heterogeneous” endometrium is described when there is heterogeneity in structure, asymmetry, or cystic formations(Fig.4).

Fig.3. “Homogeneous” endometrium: (a) schematic representation of a three-layer endometrium, (b) hypoechoic, (c) hyperechoic, (d) isoechoic.
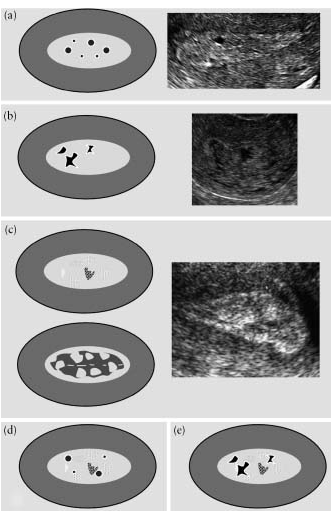
Fig.4. “Heterogeneous” endometrium: cystic formations with smooth edges are visualized against a homogeneous background (a), cystic formations with uneven edges are observed against a homogeneous background (b), a heterogeneous background without cystic areas (c), cystic formations with smooth edges are present against a heterogeneous background ( d) and on a heterogeneous background, cystic formations with uneven edges (e).
The endometrium is considered “linear” if the line of closure of the endometrial layers is determined to be straight, and “nonlinear” if the line of closure is visualized as “jagged” or “interrupted” or completely absent (Fig. 5).
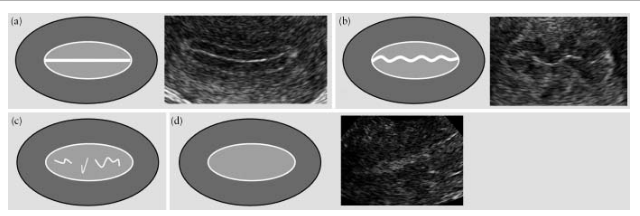
Fig.5. The line of closure of the endometrial layers: “linear” (a), “jagged” (b), “interrupted” (c) and one that is not visualized (d).
The endometrial-myometrial region is described as “smooth,” “ragged,” “interrupted,” or “indeterminate” (Fig. 6).

Fig.6. Endometrial-myometrial area: “smooth” (a), “uneven” (b), “interrupted” (c) and “indeterminate” (d).
Intrauterine fluid is described as anechoic, isoechoic, or mixed echogenicity (Fig. 7).

Fig.7. Intrauterine fluid: (a) hypoechoic, (b) isoechoic, (c) mixed echogenicity.
Doppler assessment
Doppler settings should be adjusted to ensure maximum sensitivity (ultrasound frequency at least 5.0 MHz, pulse repetition frequency (PRF) 0.3-0.9 kHz, vessel wall filter 30-50 Hz, Doppler color gain should be reduced to until all color artifacts disappear).
Doppler is scored by the presence of blood flow: a score of 1 is given when there is no flow of color signals into the endometrium, a score of 2 if only minimal flow can be detected, a score of 3 when moderate flow is present, and a score of 4 when significant blood flow is evident (Figure 8). .
DISCUSSION
We built a scoring system (REC) that can effectively distinguish benign and malignant formations endometrium. The REC scoring system correctly identified lesions in nine out of 10 postmenopausal women with endometrial thickness ≥ 5 mm. The scoring approach can be used to reduce the number of invasive procedures performed.
We used terms and classifications defined by the International Endometrial Tumor Analysis Group (IETA) that can be used to measure and describe pathology located in the uterine cavity. The main goal of this work is to create a list of terms and definitions that can be used both in the daily practice of doctors and in scientific research.
According to V.N. Demidov and A.I. Gusa, ultrasonography endometrial testing should be carried out in the first three days after the end of menstruation; normally, at this time the endometrium should be completely homogeneous and hypoechoic.
At glandular hyperplasia(GE) endometrial thickness is 1-1.5 cm, rarely reaching 2.0 cm. The echogenicity of hyperplasia is increased, the echostructure is homogeneous, often with multiple small anechoic inclusions. Sometimes an acoustic amplification effect is observed distal to the GE (Fig. 1-4). When visualizing areas of increased echogenicity against the background of a practically unchanged endometrium, it is possible to conclude that focal hyperplasia endometrium (Fig.).
The situation with ultrasound diagnostics atypical endometrial hyperplasia (AHE). A number of authors indicate that there are no specific echographic criteria for diagnosing AGE. The thickness of the endometrium in this condition ranges from 1.5-2.0 cm, in some cases reaching 3.0 cm. The echogenicity of AGE is average, the echostructure is homogeneous (Fig. 5-6).
As rightly noted by V.N. Demidov and A.I. Gus, despite the significant morphological differences in endometrial polyps (glandular, glandular-fibrous, fibrous, adenomatous), their echographic image has much in common. A typical echo picture of an endometrial polyp (PE) is an oval or round formation of medium or increased echogenicity with a clear boundary between the polyp and surrounding tissues, usually in the form of an anechoic rim (Fig. 7-15).
The size of polyps can vary very significantly, from 0.5 cm to 4-6 cm (in the case of glandular fibrous and adenomatous PE). In the presence of small PE (<0.5 см) диагностика затруднена, и, как замечают В.Н. Демидов и А.И. Гус, единственным эхопризнаком может явиться деформация срединной линейной гиперэхогенной структуры М-эхо.
Dopplerography with hyperplastic processes of the endometrium. According to B.I. Zykin, with GE, blood flow inside the mucous membrane was either not recorded (in 75-80% of patients), or a few color loci were visualized (Fig. 16).
Color Dopplerography of endometrial polyps revealed a feeding vessel in the form of a “color bridge” between the sub- and endometrial zones (Fig. 17-18).
Blood flow indicators in benign endometrial hyperplastic processes were characterized by low speed and fairly high resistance (Fig. 19-21, Table 1). Similar data were obtained by other authors.
Table No. 1. Indicators of intraendometrial blood flow during hyperplastic processes (B.I. Zykin, 2001).
Endometrial cancer
A very large number of studies are devoted to trying to correlate the risk of endometrial cancer (EC) with the thickness of the M-echo, especially in postmenopause. Thus, A. Kurjak et al consider endometrial thickness >8 mm in perimenopause and >5 mm in postmenopause to be pathognomonic for EC. S. S. Suchocki et al. did not find a single case of cancer or hyperplasia with endometrial thickness. A number of authors point out Special attention to the very low specificity of endometrial thickening as a criterion for endometrial endometriosis. So, according to I. Fistonic et al. in patients with postmenopausal bleeding, the thickness of the endometrium was: 6.2 mm with endometrial atrophy, 12.4 mm with simple hyperplasia, 13.4 mm with complex hyperplasia, 14.1 mm with carcinoma. The authors did not find significant differences in endometrial thickness between the hyperplasia and carcinoma groups. Wherein average age of patients with carcinoma was significantly higher (62 years). Bakour et al. , using an endometrial thickness of 4 mm as a criterion for malignancy, were able to diagnose endometrial carcinoma with sensitivity, specificity, PCR, PCR of 92.9%, 50.0%, 24.1%, 97.6%. The authors conclude that in women with postmenopausal bleeding, endometrial thickness<4 мм позволяет с высокой вероятностью исключить вероятность карциномы, однако толщина 4 мм не добавляет значимой информации о наличии или отсутствии малигнизации.
When diagnosing EC, an assessment of the internal echo structure of the M-echo can play a significant role. According to T. Dubinsky et al. thin homogeneous endometrium is a prognostic sign of a benign finding, while visualization of a heterogeneous echostructure always requires histological examination to clarify the diagnosis. The combined use of three echographic criteria (thickness 5 mm, uneven contour, heterogeneous echo structure) allowed G. Weber et al. diagnose endometrial carcinoma with sensitivity, specificity, PCR, PCR 97%, 65%, 80%, 94%.
The possibility of echographic assessment of malignant invasion into the myometrium is important. So according to F. Olaya et al. when diagnosing deep invasion of endometrial carcinoma into the myometrium (>50%), the sensitivity, specificity and accuracy of transvaginal echography were 94.1%, 84.8%, 88%. When differentiating the degree of invasion of endometrial carcinoma into the myometrium (no invasion, invasion of layers adjacent to the endometrium, deep invasion), the sensitivity, specificity and accuracy of transvaginal echography were 66.2%, 83.1%, 77.2%. The results obtained are comparable to the effectiveness of MRI without contrast, and slightly lower than the effectiveness of MRI with contrast.
Particularly noteworthy are the works whose authors describe cases of endometrial carcinoma in postmenopausal women with a thin or even non-visualized endometrium, or with a combination of the echo picture of endometrial atrophy and serometra (it is believed that the echo picture of fluid in the uterine cavity accompanies 50% of cases of endometrial cancer). So S. Li et al. found endometrial cancer in 3.9% of patients with endometrial thickness<5мм. По данным М. Briley и соавт. , при постменопаузальном кровотечении у 20% пациенток с невизуализируемым эндометрием имела место карцинома. Авторы считают, что у пациенток с постменопаузальным кровотечением при визуализации тонкого эндометрия (<6мм) биопсии можно избежать, однако утолщенный, и что важно - невизуализируемый эндометрий являются показанием для биопсии. H. Krissi и соавт. описали рак эндометрия при эхокартине атрофии в сочетании с серометрой, считая, что последняя может служить показанием для биопсии, поскольку компрессия стенок матки при серометре может скрывать патологические изменения эндометрия. В то же время R. Bedner и соавт. полагают, что небольшая серометра в постменопаузе (до 5 см3) вряд ли может ассоциироваться с карциномой эндометрия, описывая случай последней с объемом внутриматочной жидкости 12см3.
Moving on to detailing the echo signs of EC, it is necessary to recall that the latter is divided into pathogenetic variant I (PE-I), which occurs against the background of endometrial hyperplasia, and pathogenetic variant II, which occurs against the background of endometrial atrophy (PE-II).
- Large M-echo thickness, more than half the thickness of the uterus
- Unevenness and blurred contours
- Increased echogenicity
- Increased sound conductivity
- Heterogeneous internal echo structure
- Internal liquid inclusions
- Uneven thinning of the myometrium, indicating invasion
- Fluid in the uterine cavity. The echo picture of RE-II is completely nonspecific, but this type should be suspected if the following echo signs are found in a woman with postmenopausal bleeding (Fig. 28)
- Unvisualized endometrium
- Fluid in the uterine cavity.
|
Thus, summing up the section devoted to the echographic diagnosis of EC, one cannot but agree with B.I. Zykin, who believes that for the diagnosis of endometrial cancer, the thickness indicator is not decisive, and concludes that at the present stage, transvaginal echography (B-mode) has exhausted itself as a method for diagnosing endometrial cancer, reaching an accuracy ceiling of 75-85%.
Dopplerography for RE. As noted by B.I. Zykin, with RE-I, intraendometrial blood flow was detected in 100% of patients in the form of multiple, often chaotically located color loci (Fig. 24). Doppler indicators were characterized by high speed and low resistance of blood flow (Fig. 25-27, Table 2). Similar data have been obtained by most authors dealing with this problem.
| Figure 26 |
| Endometrial cancer (I pathogenetic variant) Low blood flow resistance |
| Figure 27 |
| Endometrial cancer (I pathogenetic variant) High blood flow speed |
In EC-II, color loci were not visualized in the projection of the atrophied mucosa, and cancer revealed itself only by a noticeable increase in blood flow in the subendometrial zones of the myometrium (Fig. 28). Thus, the only ultrasound criterion to suspect endometrial malignancy was not endometrial thickness, but abnormal color loci.
Table 2. Indicators of intraendometrial blood flow in endometrial carcinoma (B.I. Zykin, 2001).
There is no doubt that the widespread use of high-resolution transvaginal echography and Dopplerography will significantly increase the level of early detection of EC, and, possibly, reduce the frequency of unnecessary curettages in women with postmenopausal bleeding.
- Demidov V.N., Gus A.I. Ultrasound diagnosis of hyperplastic and tumor processes of the endometrium In the book: Clinical Guide to Ultrasound Diagnostics / Ed. Mitkova V.V., Medvedeva M.V. T. 3. M.: Vidar, 1997. P. 175-201.
- Demidov V.N., Zykin B.I. Ultrasound diagnostics in gynecology // M. Medicine. 1990.
- Medvedev M.V., Zykin B.I., Khokholin V.L., Struchkova N.Yu. Differential ultrasound diagnostics in gynecology // M. Vidar. 1997
- Zykin B.I. Standardization of Doppler studies in gynecological oncology // Dissertation for the degree of Doctor of Medical Sciences. Moscow. 2001. 275.P.
- Kurjak A., Kupesic S., (Ed.) An atlas of transvaginal color Doppler. Second edition. // The Parthenon publishing group. New York. London. 2000. P.161-178.
- Suchocki S., Luczynski K., Szymczyk A., Jastrzebski A., Mowlik R. Evaluation of endometrial thickness by transvaginal ultrasonography as a screening method in early diagnosis of endometrial cancer // Ginekol-Pol. 1998 May., 69(5): 279-82.
- Bakour SH., Dwarakanath LS., Khan KS., Newton JR., Gupta JK. The diagnostic accuracy of ultrasound scan in predicting endometrial hyperplasia and cancer in postmenopausal bleeding // Obstet Gynecol Scand. 1999 May., 78(5): 447-51.
- Fistonic I., Hodek B., Klaric P., Jokanovic L., Grubisic G., Ivicevic Bakulic T. Transvaginal sonographic assessment of premalignant and malignant changes in the endometrium in postmenopausal bleeding // J Clin Ultrasound. 1997 Oct., 25(8): 431-5.
- Dubinsky TJ., Stroehlein K., Abu Ghazzeh Y., Parvey HR., Maklad N Prediction of benign and malignant endometrial disease: hysterosonographic-pathologic correlation // Radiology. 1999 Feb., 210(2): 393-7.
- Weber G., Merz E., Bahlmann F., Rosch B. Evaluation of different transvaginal sonographic diagnostic parameters in women with postmenopausal bleeding // Ultrasound Obstet Gynecol. 1998 Oct., 12(4): 265-70.
- Olaya F.J., Dualde D., Garcia E., Vidal P., Labrador T., Martinez F., Gordo G. Transvaginal sonography in endometrial carcinoma: preoperative assessment of the depth of myometrial invasion in 50 cases // Eur J Radiol. 1998 Feb., 26(3): 274-9.
- Medvedev V.M., Chekalova M.A., Teregulova L.E. Endometrial cancer // In the book: Dopplerography in gynecology. Edited by Zykin B.I., Medvedev M.V. 1st edition. M. RAVUZDPG, Real Time. 2000. pp. 145-149.
- Li S., Gao S. Diagnostic value of endometrial assessment by transvaginal ultrasonography in patients with postmenopausal bleeding // Chung Hua Fu Chan Ko Tsa Chih. 1997 Jan., 32(1): 31-3.
- Briley M., Lindsell DR. The role of transvaginal ultrasound in the investigation of women with post-menopausal bleeding // Clin Radiol. 1998 Jul., 53(7): 502-5.
- Krissi H., Bar Hava I., Orvieto R., Levy T., Ben Rafael Z. Endometrial carcinoma in a post-menopausal woman with atrophic endometrium and intra-cavitary fluid: a case report // Eur J Obstet Gynecol Reprod Biol. 1998 Apr., 77(2): 245-7.
- Bedner R., Rzepka Gorska I. Diagnostic value of uterine cavity fluid collection in the detection of pre-neoplastic lesions and endometrial carcinoma in the asymptomatic post-menopausal women // Ginekol Pol. 1998 May., 69(5): 237-40.
Copyright © 2000-2006 "Iskra Medical Corporation", Bulanov M.N.
All rights reserved. No part of this page (including text, illustrations and files) may be reproduced in any form or by any means without the written permission of the copyright owners.According to world statistics, uterine cancer ranks 7th among malignant diseases. An analysis of the oncological situation in the last decade in Russia indicates a steady increase in the incidence of endometrial cancer, which by 2007 took 2nd place among all malignant tumors in women. The share of uterine cancer in the structure of the incidence of malignant neoplasms per 100,000 female population of Russia in different regions ranges from 4.5 to 22.5. There is a steady increase in the incidence rate from 9.8 in 1990 to 13.9 in 2005, which corresponds to the 3rd place in terms of the increase in the incidence of malignant neoplasms. Currently, the increase in the number of newly diagnosed cases of uterine cancer is not inferior to that of breast tumors. In third world countries, the risk of developing endometrial cancer is generally lower, but the mortality rate remains high. In North America and Europe, this disease is much more common, being the most common malignant tumor of the female reproductive system, and ranks 4th among all malignant neoplasms after breast, lung and colon cancer. The incidence of endometrial cancer increases sharply between the ages of 40 and 54 years, with the peak incidence occurring between the ages of 60 and 64 years. The incidence of endometrial cancer and its dynamics in different countries, taking into account the influence of migration processes and age, indicate the specific features of the disease and the dependence of its occurrence on a complex of causes of endo- and exogenous nature.
Among the risk factors for the development of uterine cancer, attention is drawn to a low number of births or infertility, obesity, late menopause, and diabetes mellitus, mainly type 2. In most cases, the risk of developing endometrial cancer is associated with various forms of endometrial hyperplasia - 81.3%, dysfunction due to polycystic ovary syndrome - 25%, endometrial polyposis - 5.3-25%, uterine fibroids - 1.6-8%. Recently, there has been a significant increase in locally advanced forms of endometrial cancer, which is associated with ineffective primary diagnostic measures. Issues of clarifying diagnosis of endometrial cancer are the subject of close study.
In the pathogenesis of the disease, the theory of excessive estrogen stimulation of the endometrium, combined with progesterone deficiency, is of leading importance. It is believed that excessive estrogen exposure can lead to endometrial hyperplasia, which can progress to an atypical variant and in 20-25% of cases to transition to adenocarcinoma. At the same time, the existing relationship between the degree of endometrial proliferation and the concentration of estrogen in the blood is observed up to a certain threshold value, and even intense proliferation is not in all cases accompanied by malignant transformation of the endometrium. The discovered correlation between estrogen content and DNA damage in normal and malignant endometrium forces us to pay more attention to the role of molecular genetic and morphological factors in the formation of different types of uterine cancer. Endometrial cancer is characterized by a heterogeneous nature, which manifests itself at the level of both risk factors and its pathogenesis, which determines the features of the formation of risk groups for this disease.
Currently, to identify endometrial pathology, diagnostic curettage of the uterine cavity, hysteroscopy and aspiration cytological examination, as well as radiation diagnostic methods, among which ultrasound (ultrasound) is of leading importance, are mainly used. However, there are no uniform methodologically based echographic criteria for invasive tumor growth. The introduction of new ultrasound technologies into programs for integrated examination of patients, such as pulsed Doppler, ultrasound angiography and three-dimensional image reconstruction, has significantly increased the efficiency of primary diagnosis and monitoring of patients with endometrial cancer in the process of specific therapy.
The purpose of this work was to study the capabilities of complex ultrasound using color Doppler and (and EC), and three-dimensional image reconstruction in the primary and clarifying diagnosis of endometrial cancer.
Material and methods
We examined 139 patients aged from 21 to 87 years with suspected endometrial cancer in the peri- and postmenopausal period. In 34 patients, hyperplastic processes of the endometrium were detected, in 105 - malignant processes of the endometrium. The average age of patients with benign pathology was 42.6±7.2 years, of patients with endometrial cancer - 65.4±7 years. In all cases, histological verification of the diagnosis was obtained.
All patients were comprehensively examined by ultrasound using transabdominal (3.5 MHz convex sensor) and transvaginal (6.5-7 MHz sensor) approaches on modern ultrasound machines Logiq S6 (GE, Healthcare) and Accuvix-XQ (Medison) according to a certain program using the latest ultrasound techniques, including Doppler ultrasound of the uterine vessels, color circulation and EC with three-dimensional image reconstruction. During transabdominal examination in patients with a full bladder, the condition of the uterus and ovaries was assessed, the volumes of the body and cervix, and the width of the M-echo were determined. During transvaginal ultrasound (TVUS), Doppler measurements were used to measure blood flow and resistance index in the uterine arteries, and to evaluate the intensity of intratumoral blood flow. At all stages of the study, the state of the endo- and myometrial structure, their relationship and homogeneity were determined. When focal changes were detected, their size, degree of prevalence and relationship with surrounding organs and structures were determined, and a comparative assessment of the thickness of the uterine wall in the tumor area and outside the tumor focus area was carried out. If possible, the linear and volumetric parameters of the tumor, the clarity of its contours were accurately determined, and the condition of the adjacent mucous membrane was assessed. The main parameter for assessing the endometrium remains the change in its thickness. Endometrial volume is also used for the earliest diagnosis of the disease. Its values are more reliable in the differential diagnosis of cancer and benign hyperplastic processes than measurements of endometrial width. The criteria for malignant endometrial lesions are endometrial volume values exceeding 13 cm 3 . This ensures 100% sensitivity and 92% predictability of a positive test in the diagnosis of endometrial cancer.
The most important characteristics of an endometrial neoplasm were the degree and nature of its vascularization, which was assessed in the cine-loop mode in order to obtain the most complete and visual representation. A qualitative assessment of blood supply was carried out according to the number of color signals from the vessels of the neoplasm: hypovascular, moderately vascular, hypervascular. We used a technology that converted volumetric data into a series of successive sections up to 0.5 mm thick. The targeted selection of certain sections from 3D volumetric data made it possible to select optimal sections of the body and uterine cavity and most accurately estimate their sizes, determine the relationship of the identified changes with the condition of surrounding organs and tissues. Volume CT View technology made it possible, based on 3D scanning, to evaluate the contours and structure of the endometrium, the nature of its blood supply, and the use of the histogram option made it possible to accurately determine the vascularization index.
Particular attention was paid to assessing the depth of myometrial invasion, the possible transition of the malignant process to the cervical canal and the condition of the regional lymph nodes, which was crucial in determining the stage of the disease and choosing treatment tactics.
Results and discussion
As a result of the study, endometrial hyperplastic processes were identified in 34 patients, which we identified into separate nosological forms that comply with WHO recommendations. In table Figure 1 shows the distribution of patients depending on the morphogenesis of the identified endometrial hyperplastic processes.
Table 1. Distribution of patients according to the type of endometrial hyperplastic processes.
Hyperplastic processes of the endometrium were manifested by menstrual cycle disorders such as menometrorrhagia, anemia of I-II degrees. In case of benign endometrial pathology, in 24 (71.4%) patients, an increase in the thickness of the M-echo on average was determined by gray scale ultrasound of 14.6 ± 3.2 mm. With transvaginal echography, glandular cystic hyperplasia was defined as the formation of increased echogenicity, a homogeneous structure, with multiple point hypo- or anechoic inclusions up to 1.5 mm, sometimes with an acoustic amplification effect. With atypical hyperplasia, a heterogeneous hyperechoic solid structure was detected in the uterine cavity. Polyps were defined as round, oval or oblong, in some cases on a long stalk, hyperechoic formations of various sizes, deforming the uterine cavity and clearly differentiated against the background of the liquid contents of the uterine cavity. Using pulsed Doppler mode, hemodynamic parameters in the uterine arteries were quantitatively assessed, which were: MSS - 9.3±2.1 cm/s, resistance index - 0.56±0.05.
Using the technique, color intratumoral blood flow in glandular cystic hyperplasia was recorded in the form of single signals from vessels located along the periphery. In fibroglandular polyps, moderately pronounced venous and arterial peripheral blood flow with average values of peripheral vascular resistance was visualized. In 2 patients with glandular hyperplasia, pronounced hypervascularization of the endometrium was determined. In atypical hyperplasia, central and peripheral intratumoral blood flow of moderate intensity was recorded. In 5 patients with glandular cystic hyperplasia and atrophic endometrium, blood flow was not recorded. Characteristic signs of a benign neoplasm, even in the presence of multiple polypoid growths, were the preservation of the shape of the uterine cavity, a clear definition of the outer contour of the endometrium and a uniform distribution of myometrial vessels (Fig. 1 and 2).
Rice. 1. TVUS, energy mapping mode. Glandular cystic endometrial hyperplasia.

Rice. 2. TVUS, energy mapping mode. Endometrial polyp.
Malignant endometrial pathology was diagnosed in 105 patients. 80% of those examined with this pathology were aged from 50 to 69 years, of which 82 (78%) had malignant transformation of the endometrium accompanied by postmenopausal bleeding. An examination of patients with suspected endometrial cancer revealed an increase in the thickness of the M-echo to 18.1±6.7 mm. At stage Ia, the thickness of the M-echo was 11.5±3.7 mm, at stage Ib - 15.8±8.4 mm, at stage Ic - 17±3.4 mm, at stage II - 21±4.1 mm, at stage III - 27±2.0 mm, at stage IV - more than 30 mm. The stage of uterine cancer was determined according to the International Classification of Cancer (FIGO, 1988). In table 2, a comparison was made of a specific histotype of endometrial cancer with the stage of the disease.
table 2. Comparison of histotype and stage of endometrial cancer.
| Tumor histotype | Stage | Total | |||||
|---|---|---|---|---|---|---|---|
| Ia | Ib | Ic | II | III | IV | ||
| Adenocarcinoma: | |||||||
| highly differentiated | 12 | 3 | 3 | 4 | 2 | 1 | 25 |
| moderately differentiated | 22 | 6 | 2 | 6 | 6 | 2 | 44 |
| low-grade | 5 | - | - | 1 | 3 | 1 | 10 |
| serous-papillary | 3 | - | - | 1 | 3 | 1 | 8 |
| clear cell | - | 1 | - | - | - | 1 | 2 |
| Glandular squamous cell carcinoma | 1 | 1 | - | 2 | 1 | - | 5 |
| Sarcoma | 2 | - | 1 | 1 | 3 | 2 | 9 |
| Acanthoma | 1 | 1 | - | - | - | - | 2 |
| Total | 46 | 12 | 6 | 15 | 18 | 8 | 105 |
As can be seen from table. 2, more than 60% of patients were diagnosed with stage I uterine cancer, with 46 patients having stage Ia. Patients with common forms of malignant diseases of the uterine body accounted for 23%. In most cases (89 patients, 85%), adenocarcinoma of varying degrees of differentiation was diagnosed.
In our study, the degree of tumor differentiation correlated with the stage of the disease: in highly differentiated adenocarcinoma, the process was mainly limited to the uterine body. Poorly differentiated, serous papillary and clear cell adenocarcinomas were noted at stages II, III and IV with tumor spread beyond the organ. Stage I squamous cell carcinoma was diagnosed in 2 patients, stages II and III - in 3. A combination of adenocarcinoma and endometrial stromal sarcoma was detected in 9 patients, of which 5 were diagnosed with stages III and IV of the disease. The main ultrasound signs of endometrial cancer during transabdominal and transvaginal studies in B-scan mode can be considered an increase in M-echo, which is not typical for this patient, unevenness and heterogeneity of the endometrium, in addition, a higher echogenicity of its structure as a whole or the identified focal formation compared with unchanged myometrium, the presence of an uneven, external contour penetrating into different depths. In cases of significant local spread of the tumor, visualization of a hypoechoic rim around the tumor or the absence of a border between the tumor focus and the myometrium is possible. In our study, we assessed the invasive growth index (IGI) - determining the ratio of the volume of the altered endometrium (AVI) to the volume of the uterine body. The obtained data are presented in table. 3. Calculation of these indicators was possible only for stage I endometrial cancer, when the border of the altered endometrium was defined quite clearly (Fig. 3).
Table 3. Ultrasound parameters of the uterus and M-echo in endometrial cancer of different stages.
At stage Ia, the volume of the endometrium was 4.2±2.2 cm3, IIR - 11.9±4.2, at stage Ib AIE - 8.3±4.6 cm3, IIR - 7.5±5.4 cm3, at stage Ic AIE - 15.4±5.3 cm3, IIR - 4.3±2.9. As shown in the table. 3 data, there is a clear increase in endometrial volume and a decrease in IRI values as the degree of tumor invasion in the myometrium increases. For the majority of patients with endometrial cancer, its localization was characteristic of the fundus of the uterus or one of the tubal angles. Tumor necrosis with deformation of the uterine cavity and the presence of fluid in it were determined at stages III and IV of the process.
Based on literature data, we have identified three main types of growth of invasive endometrial cancer.
- Development of multiple highly differentiated tumor foci against the background of hyperplastic processes of the entire endometrium.
- Development of one highly differentiated tumor focus, surrounded by a hyperplastic mucous membrane over a short area.
- Development of one moderately or poorly differentiated tumor focus against the background of an atrophic mucous membrane.
The exophytic form of tumor growth was detected in 15% of cases. Exophytic tumor growth is characterized by the absence of deformation of the uterine cavity, clear boundaries of the endo- and myometrium, or the detection of a formation in the lumen of the uterine cavity. In 85% of cases, an endophytic form of growth with invasion into the myometrium was noted. Violation of the integrity of the hypoechoic rim in endometrial cancer is a specific sign of invasion into the myometrium. Endophytic tumor growth leads to asymmetry and deformation of the uterine cavity. With a deep infiltrative process, the 2nd option was noted in 30%, the 3rd option - in 70% of cases. Ultrasound made it possible to clearly determine the shape of tumor growth only in the initial stages of the disease. In stage Ia endometrial cancer, in the case of ultrasound in B-mode, a homogeneous hyperechoic structure of the median M-echo was determined, and in 69.5%, heterogeneity of the endometrial structure was revealed due to inclusions of a round shape, with smooth, in some cases unclear contours, increased echogenicity, the average size of which was 6.3±3.8 mm. The boundaries of the endometrium in all observations at stage I of the disease were determined to be clear and even.
Table 4. Hemodynamic parameters in benign and malignant pathology of the endometrium.
Note. * - R<0,05
In our observations, it was possible to differentiate the 1st and 2nd types of tumor development in the usual B-mode only in 10 patients. In other cases, due to significant local spread of the tumor, these differences were not determined. With a deep infiltrative process at stages III and IV of the disease, the thickness of the M-echo exceeded 27.0 mm. The boundaries between the tumor and myometrium in all cases were unclear, the contours were uneven, and in 61 (58.0%) patients the tumor boundaries were not defined up to the outer contour of the uterus. The structure of the M-echo in 30.3% of cases was homogeneous hyperechoic, in 20.1% - homogeneous hypoechoic, and in 50% - heterogeneous, predominantly hyperechoic. The echostructure of the tumor could also have different echogenicity: in 44.6% of cases it was homogeneous hyperechoic, in 10.4% - homogeneous hypoechoic, in 45.0% - mixed.
We assessed the quantitative indicators of hemodynamics, performed using the uterine arteries and tumor vessels. In table Table 4 shows a comparative description of hemodynamic parameters in benign and malignant endometrial pathologies.
As can be seen from the data presented, the hemodynamics of regional blood flow in endometrial cancer is accompanied by a tendency to increase the speed of blood flow in the vessels of the uterus and a statistically significant decrease in the index of peripheral resistance in tumor vessels, which can characterize the activity of intratumoral blood flow. MSS in the uterine arteries depended on the volume of the uterine body, which could be associated with the presence of fibroids, and the nature of tumor vascularization. The indicators of intratumoral blood flow and IR were not statistically dependent on the histotype of endometrial cancer.

Rice. 4. TVUS, energy mapping mode. Endometrial cancer stage Ia. A focus of hypervascularization is identified along the anterior wall of the uterus.

Rice. 5.

Rice. 6. TVUS, Color Doppler, Longitudinal Scanning. Endometrial cancer stage Ia. Infiltrative formation of a hyperechoic structure in the area of the fundus of the uterine body with reduced vascularization.
When analyzing the nature and degree of vascularization of endometrial cancer, assessed using the CDC and EC modes, different options for intraendometrial blood flow were determined. Pathological vascularization of the endometrium occurred in 92 (87.6%) patients with endometrial cancer. In other cases, even in the presence of characteristic ultrasound signs of malignant lesions, intratumoral blood flow was not visualized using the techniques used. In case of a tumor of the uterine body, three main variants of blood supply were identified (A, B, C), and a certain dependence of the pattern of color circulation and EC with the stages and identified forms of tumor growth was observed. The intensity of blood flow in the endometrium and tumor node, determined in the CDC and EC modes, depended on the type of tumor growth and could be most clearly presented in the cine-loop mode. Zones of tumor blood flow are detected in endometrial cancer in more than 90% of cases (Fig. 4-8).
It was established that option A was characteristic of stage Ia: with infiltration of the myometrium to a depth of 5 mm, which was determined in 33.8% of cases and was characterized by an uneven increase in intraendometrial blood flow due to a local increase in the number of color spots with different color intensities, in the absence of color loci in the subendometrial zone. The same variant was characteristic of the exophytic form of growth with an intratumoral type of neovascularization.

Rice. 7. TVUSI, CDC. Stage IV endometrial cancer. Hypervascularization of the formation of a heterogeneous structure in the area of the left uterine angle. Endometrial and intratumoral blood flow is determined.

Rice. 8. TVUS, combination of B-mode and energy mapping mode. Stage IV endometrial cancer. A focus of hypervascularization along the posterior wall of the uterus with hypervascular endometrial blood flow.
Option B (47.6%) was characterized by a total increase in intraendometrial blood flow due to a large number of chaotically located color loci, with a simultaneous local increase in the number of color signals in the subendometrial zone. In 27.5% of cases, moderate vascularization of the tumor was determined, combined with rich vascularization of the myometrium. This variant was identified in 78.3% of patients with a mixed form of endometrial cancer.
Option C (19.6%) was characterized by a slight increase in intraendometrial blood flow with a significant total increase in the number of color signals in the subendometrial zone. This variant was characteristic of the endophytic growth form (92.5%) and was accompanied by intense intra- and peritumoral blood flow.
Although no direct correlation was established between the severity of tumor blood flow and the stage of the disease, as well as the degree of differentiation, the presence of a detectable zone of neovascularization corresponded to a higher stage of the process. Hypovascular and moderate blood flow in the endometrium was observed in patients with well-differentiated adenocarcinoma.
Neovascularization of the pathological process was not registered in 12.4% of cases. The reason for this could be the removal of a small tumor as a result of preliminary diagnostic curettage of the uterine cavity and in highly differentiated adenocarcinoma that arose against the background of endometrial atrophy.
By constructing frontal planes, it made it possible to more accurately determine the state of the endometrium and establish its asymmetry. A disorganized vascular pattern, revealed by three-dimensional angiography in a volumetric block, when combining scanning modes, was an important additional sign of malignant endometrial lesions. The most accurate results in assessing the degree of invasion of endometrial carcinoma are achieved using three-dimensional reconstruction in ultrasound angiography mode (Fig. 9-11). An important sign of common invasive processes is the presence of zones of local increased vascularization in the myometrium adjacent to the tumor areas.

Rice. 9. Ultrasound performed using Multi-slice view technology. Using layer-by-layer sections, it becomes possible to accurately determine the structure of the endometrium and its vascularization.
The capabilities of the ultrasound method in diagnosing endometrial cancer have their limitations due to the fact that hyperplastic processes and the initial stages of the disease do not have specific differential diagnostic features. Concomitant uterine bleeding with the formation of fibrin complicates the identification of areas of endometrial thickening. Certain difficulties arise in determining the depth of myometrial invasion in the initial stages of endometrial cancer within limits of up to 5 mm, as well as in cases of concomitant adenomyosis. Ultrasound does not accurately determine the volume of cancerous lesions in women with large and multiple submucosal myomatous nodes that deform the uterine cavity.

Rice. 10. TVUS, energy mapping mode. Ultrasound performed using Oblique view technology. 3D volumetric data allows us to clarify the state of the endometrium and the nature of endometrial and subendometrial vascularization.

Rice. eleven. Multi-plane reconstruction mode. Volume CT view. 3D data allows you to determine volumes as accurately as possible.
conclusions
Ultrasound examination using pulsed Doppler, color Doppler, energy mapping and three-dimensional image reconstruction is a highly informative method for non-invasive clarifying diagnosis of endometrial pathology. The results obtained indicate the high efficiency of the methods used in the differential diagnosis of benign and malignant processes. Ultrasound angiography and three-dimensional image reconstruction for endometrial cancer help obtain additional and very important information about the characteristics of the tumor process, the depth of tumor invasion into the myometrium, and the nature of the detected neovascularization makes it possible to predict the rate of tumor growth.
The use of modern ultrasound technologies makes it possible to solve the problems of intranosological diagnosis of endometrial cancer at a completely new qualitative and quantitative level, as well as to monitor patients in the process of specific treatment.
Literature
- Davydov M.I., Aksel E.M. Statistics of malignant neoplasms in Russia and the CIS countries in 2005 // Bulletin of the Russian Oncological Research Center named after. N.N. Blokhin RAMS. 2007. T. 18.
- Urmancheeva A.S., Tyulyandin S.A., Moiseenko V.M. Practical oncogynecology (Selected lectures) // M.: Publishing house. "Tomm Center" 2008. 400 p.
- Ashrafyan L.A., Kharchenko N.V., Ogryzkova V.L. and others. Modern principles of primary and clarifying diagnosis of endometrial cancer // Practical Oncology. 2004. T. 5. No. 1.
- Demidov V.N., Tue A.I. Ultrasound diagnosis of hyperplastic and tumor processes of the endometrium // Ed. Mitkova V.V., Medvedeva M.V. Clinical guide to ultrasound diagnostics, 3 vols. M.: Vidar. 1997. pp. 120-131.
- Kapustina I.N., Sidorova A.N., Sarantsev A.N. Color Doppler mapping in the diagnosis of endometrial cancer // Sonoace international. Russian version. Vol. 9, 2001. pp. 34-39.
- Maksimova N.A. Some aspects of ultrasound diagnosis of endometrial cancer // Ultrasound diagnosis in obstetrics, gynecology and pediatrics. 1999. No. 3. P. 196-201.
- Stolyarova I.V., Minko B.A., Sirazitdinov B.R. Capabilities of three-dimensional ultrasonic angiography in specify diagnostics of endometrial carcinoma // 19 International Congress on Anti Cancer Treatment Paris, February 5-8; 2008. P. 255-256.
- Gazhenova V.E. Ultrasound diagnostics in gynecology // M.: "MEDpress-inform". 2005. 264 p.
- Titova V.A., Kharchenko N.V., Stolyarova I.V. Automated radiation therapy of the female reproductive system // M.: Medicine. 2006. 160 p.
- Gruboeck K., Jurkovic D., Lawton F. et al. The diagnostic value of endometrial thickness and volume measurements by three-dimensional ultrasound in patients with postmenopausal bleeding // Ultrasound Obstet. Gynecol. 1996. No. 8. P. 272-276.
- Stolyarova I.V., Minko B.A., Lisyanskaya A.S. and others. Possibilities of modern ultrasound techniques in the clarifying diagnosis of endometrial cancer // International Congress Nevsky Radiological Forum "New Horizons" April 7-10, 2007 St. Petersburg, pp. 364-365.
- Chekalova M.A., Zuev V.M. Ultrasound diagnostics in gynecological oncology // M.: Publishing House. house "Russian doctor". 2004. 92 p.
- Teregulova A.E. Transvaginal echography using color Doppler mapping in patients with endometrial cancer // Ultrasound diagnostics. 1996. No. 4. pp. 21-23.
- Kurjak A., Shalan H., Sosic A. et al. Endometrial carcinoma in postmenopausal women: evaluation by transvaginal color Doppler ultrasonography // Am. J. Obstet. Gynecol. 1993. V. 169. P. 1597-1603.








