Saliva (saliva) is the secretion of the salivary glands, secreted into the oral cavity. In the oral cavity there is a biological fluid called oral fluid, which, in addition to the secretion of the salivary glands, includes microflora and its waste products, the contents of periodontal pockets, gingival fluid, desquamated epithelium, leukocytes migrating into the oral cavity, food residues, etc. Oral fluid is a viscous liquid with a relative density of 1.001-1.017.
An adult produces 1500-2000 ml of saliva per day. However, the rate of secretion varies depending on a number of factors: age (after 55-60 years, salivation slows down), nervous excitement, food irritant. During sleep, saliva is secreted 8-10 times less - from 0.5 to 0.05 ml/min than during wakefulness, and during stimulation - 2.0-2.5 ml/min. With a decrease in salivation, the degree of dental caries damage increases. In practice, the dentist deals with oral fluid, since it is the environment in which the organs and tissues of the oral cavity are constantly located.
The buffering capacity of saliva is the ability to neutralize acids and bases (alkalies), due to the interaction of hydrocarbonate, phosphate and protein systems. It has been established that eating carbohydrate foods for a long time reduces, and eating high-protein foods increases the buffer capacity of saliva. The high buffering capacity of saliva is one of the factors that increases the resistance of teeth to caries.
The concentration of hydrogen ions (pH) has been studied in some detail, which is due to the development of Miller's theory about the occurrence of dental caries. Numerous studies have established that the average pH of saliva in the oral cavity is normal conditions is in the range of 6.5-7.5. Slight fluctuations in pH were found during the day and night (decrease at night). The most powerful factor that destabilizes the pH of saliva is acid-producing activity after ingestion of carbohydrate foods. "Sour" reaction oral fluid observed very rarely, although a local decrease in pH is a natural phenomenon and is caused by the vital activity of the microflora of dental plaque, carious cavities, and saliva sediment.
Composition of saliva and oral fluid. Saliva consists of 99.0-99.4% water and 1.0-0.6% organic minerals dissolved in it. Of the inorganic components, saliva contains calcium salts, phosphates, potassium and sodium compounds, chlorides, bicarbonates, fluorides, rhodanites, etc. The concentration of calcium and phosphorus is subject to significant individual fluctuations (1: -2 and 4-6 mmol/l, respectively), which are mainly located in bound state with saliva proteins. The calcium content in saliva (1.2 mmol/l) is lower than in blood serum, and phosphorus (3.2 mmol/l) is 2 times higher. Oral fluid also contains fluoride, the amount of which is determined by its intake into the body.
The ionic activity of calcium and phosphorus in oral fluid is an indicator of the solubility of hydroxy- and fluorapatites. It has been established that saliva under physiological conditions is supersaturated in hydroxyapatite (ion concentration 10"117) and fluorapatite (10"w), which allows us to speak of it as a mineralizing solution. It should be noted that the oversaturated state under normal conditions does not lead to the deposition of mineral components on the surfaces of teeth. Proline- and tyrosine-rich proteins present in oral fluid inhibit spontaneous precipitation from solutions supersaturated with calcium and phosphorus.
It is noteworthy that the solubility of hydroxyapatite in oral fluid increases significantly with a decrease in its pH. The pH value at which oral fluid is saturated with enamel apatite is considered a critical value and, according to calculations confirmed by clinical data, varies from 4.5 to 5.5. At pH 4.0-5.0, when the oral fluid is not saturated with both hydroxyapatite and fluorapatite, the surface layer of enamel dissolves according to the type of erosion (Larsen et al.). In cases where saliva is not saturated with hydroxyapatite, but is oversaturated with fluorapatite, the process follows the type of subsurface demineralization, which is characteristic of caries. Thus, the pH level determines the nature of enamel demineralization.
The organic components of oral fluid are numerous. It contains proteins synthesized both in the salivary glands and outside them. The salivary glands produce enzymes: glycoproteins, amylase, mucin, as well as class A immunoglobulins. Some salivary proteins are of serum origin (amino acids, urea). Species-specific antibodies and antigens that make up saliva correspond to the blood group. Up to 17 protein fractions of saliva were isolated by electrophoresis.
Enzymes in mixed saliva are represented by 5 main groups: carbonic anhydrases, esterases, proteolytic, transfer enzymes and a mixed group. Currently, there are more than 60 enzymes in oral fluid. Based on their origin, enzymes are divided into 3 groups: secreted by the parenchyma salivary gland, formed during the enzymatic activity of bacteria, formed during the breakdown of leukocytes in the oral cavity.
Of the salivary enzymes, first of all, L-amylase should be isolated, which in the oral cavity partially hydrolyzes carbohydrates, converting them into dextrans, maltose, mannose, etc.
Saliva contains phosphatases, lysozyme, hyaluronidase, kininogenin (kallikrein) and kallikrein-like peptidase, RNase, DNase, etc. Phosphatases (acidic and alkaline) participate in phosphorus-calcium metabolism, splitting phosphate from phosphoric acid compounds and, thereby, providing mineralization of bones and teeth. Hyaluronidase and kallikrein change the level of tissue permeability, including tooth enamel.
The most important enzymatic processes in the oral fluid are associated with the fermentation of carbohydrates and are largely determined by the quantitative and qualitative composition of the microflora and cellular elements of the oral cavity: leukocytes, lymphocytes, epithelial cells and etc.
Oral fluid, as the main source of calcium, phosphorus and other mineral elements into tooth enamel, affects the physical and Chemical properties tooth enamel, including resistance to caries. Changes in the quantity and quality of oral fluid are important for the occurrence and course of dental caries.
Functions of saliva
Saliva plays a huge role in maintaining the normal condition of the organs and tissues of the oral cavity. It is known that with hyposalivation, and especially xerostomia (lack of saliva), inflammation of the oral mucosa quickly develops, and after 3-6 months multiple dental caries damage occurs. The lack of oral fluid makes it difficult to chew and swallow food. The functions of saliva are varied, but the main ones are digestive and protective.
The digestive function is primarily expressed in the formation and primary processing of the food bolus. In addition, food in the oral cavity undergoes primary enzymatic processing; carbohydrates are partially hydrolyzed under the action of L-amylase to dextrans and maltose.
Protective function. It is carried out thanks to the diverse properties of saliva. Moisturizing and covering the mucous membrane with a layer of mucus (mucin) protects it from drying out, cracking and exposure to mechanical irritants. Saliva washes the surface of the teeth and the mucous membrane of the mouth, removing microorganisms and their metabolic products, food debris, and detritus. The bactericidal properties of saliva, expressed through the action of enzymes (lysozyme, lipase, RNase, DNase, opsonins, leukins, etc.), are important.
The coagulating and fibrinolytic ability of saliva is supported by the thromboplastin, antiheparin substance, prothrombins, fibrinolysin activators and inhibitors it contains. These substances have hemocoagulating and fibrinolytic activity, which ensures local homeostasis and improves the regeneration processes of damaged mucous membranes. Saliva, being a buffer solution, neutralizes acids and alkalis entering the oral cavity. Finally, immunoglobulins present in saliva play an important protective role.
Mineralizing effect of saliva. This process is based on mechanisms that prevent the release of its components from the enamel and facilitate their entry from saliva into the enamel.
Calcium in saliva is in both ionic and bound states. It is believed that on average 15% of calcium is associated with proteins, about 30% is in complex bonds with phosphates, citrates, and only 5% is in the ionic state. It is this ionized calcium that is involved in remineralization processes.
It has now been established that oral fluid under normal conditions (pH 6.8-7.0) is oversaturated with calcium and phosphorus. With a decrease in pH, the solubility of enamel hydroxyapatite in oral fluid increases significantly.
For example, at pH 6.0, oral fluid becomes calcium-deficient. Thus, even minor fluctuations in pH, which are not capable of causing demineralization on their own, can actively influence the maintenance of the dynamic balance of tooth enamel.
The physicochemical constancy of enamel depends entirely on the composition and acid-base balance of the oral fluid. The main factor in the stability of enamel apatites in saliva is the pH and concentration of calcium, phosphate and fluoride compounds.
Oral fluid is a labile environment, and its quantitative and qualitative composition is influenced by many factors and conditions, but primarily by the state of the body. With age secretory function the large and small salivary glands decrease. Impaired salivation also occurs in acute and some chronic diseases. Thus, with foot and mouth disease, excessive secretion of saliva develops (up to 7-8 liters per day), which is one of the important diagnostic signs. With hepatocholecystitis, on the contrary, hyposalvation is noted, and patients complain of dry mouth. At diabetes mellitus the glucose content in the oral fluid increases.
The hygienic condition of the oral cavity has a great influence on the composition and properties of oral fluid. Deterioration in oral care leads to an increase in plaque on the teeth, an increase in the activity of a number of enzymes (phosphatease, aspartic transaminase), an increase in salivary sediment, and the rapid proliferation of microorganisms, which creates conditions, especially when frequent use carbohydrates, for production organic acids and pH changes.
Anticarious effect of saliva. It was found that soon after solid carbohydrate food enters the oral cavity, the concentration of glucose in saliva decreases, first quickly and then slowly. In this case, the speed of salivation plays a great role - increased salivation contributes to more active leaching of carbohydrates. In this case, there is no removal of fluorides, since they bind to the surfaces of the hard and soft tissues of the oral cavity, being released within a few hours. Due to the presence of fluorides in saliva, the balance between de- and remineralization shifts towards the latter, which provides an anti-caries effect. It has been established that this mechanism is realized even at relatively low concentrations of fluorides in saliva.
The effect of saliva on accelerating the excretion of glucose is not the only mechanism for reducing the incidence of caries. A more pronounced anti-caries effect is ensured by its ability to neutralize acids and alkalis, i.e., a buffer effect due to the presence of sodium bicarbonates.
Saliva is normally oversaturated with calcium, phosphorus and hydroxydapatite ions, the compounds of which form the basis of tooth tissue. The degree of supersaturation is even higher in the liquid phase of plaque, which is in direct contact with the tooth surface. The supersaturation of saliva with ions, which form the basis of dental tissues, ensures their entry into the tissues, i.e. driving force mineralization. When the pH of dental plaque decreases, the supersaturated state of saliva with calcium, phosphorus and hydroxyapatite ions decreases and then disappears altogether.
A number of salivary proteins are also involved in the remineralization of subsurface layers of enamel. Molecules of statherin and acidic, proline-rich proteins, as well as some phosphoproteins that bind calcium when the pH in plaque decreases, release calcium and phosphorus ions into the liquid phase of plaque, which supports remineralization.
Other anti-carious mechanisms include the formation of a film (pellicule) on the surface of the enamel of salivary origin. This film prevents direct contact of the enamel with acids entering the oral cavity and, thereby, prevents the release of calcium and phosphorus from its surface.
An important and least constant parameter of homeostasis is the acid-base balance in the oral cavity. The most informative indicator of acid-base balance is the pH value. This indicator varies depending on the area of the cavity: the pH value is acidic in the interdental spaces and neutral or slightly alkaline at the tip of the tongue. An integral indicator of acid homeostasis in the oral cavity is the pH of saliva. Normally, the pH of saliva is in the range of 6.5-7.5.
Changes acid-base balance in the oral cavity there can be two types: acidosis or alkalosis. In any direction of shifts in homeostasis, physiological and pathological changes should be distinguished. Physiological changes are short-term, do not lead to disruption of normal physiological processes and do not affect the structure and function of oral tissues. Pathological changes significantly exceed the boundaries of the norm and lead to disturbances in the structure and functions of certain tissues of the oral cavity: caries, desquamation of the mucosal epithelium, tartar deposition, periodontitis.
Many endo- and exogenous factors influence the acid-base balance in the oral cavity: the general condition of the human body, the severity of conditioned and unconditioned reflexes, muscle (chewing) activity, breathing pattern, speech, food, oral microflora, hygiene products, dentures, fillings and more. The most pronounced influence in physiological conditions is the vital activity of microflora, the composition of food, the composition and rate of saliva secretion.
Raid
Acid-base balance in the oral cavity depends on the presence of plaque.
Microbial plaque Forms mainly on the surfaces of teeth, artificial dentures and on the back of the tongue. Dental plaque (dental plaque)- an accumulation of microorganisms living in the oral cavity on the surface of the teeth with the inclusion of structureless substances of organic nature: proteins, lipids, carbohydrates. Among carbohydrates important has dextran - a homooligosaccharide consisting of glucose residues. Dextran has the ability to adhere (sorb) bacteria to dental plaque. Mature dental plaque contains about 2.5 10 11 bacteria in 1 g.
The main source of energy production from plaque bacteria is the processes of anaerobic breakdown of carbohydrates: lactic acid, butyric acid, propionic acid fermentation. Lactate and other organic acids produced by microbial plaque during the utilization of food carbohydrates are the main “culprits” of acidotic changes not only in the area of dental plaque, but also in the oral fluid. In plaque, there is a process of utilization of urea, which enters the oral cavity mainly with saliva. Bacterial ureases break down urea into ammonia and carbon dioxide. Ammonia, by binding protons, shifts the acid-base balance to the basic side. However, this is not enough to counteract the powerful “metabolic explosion” caused by carbohydrates.
Food
Acid-base balance in the oral cavity depends on food. Food is a destabilizer of acid-base balance. The influence of food should be considered from several aspects.
First, food contains acids and bases. Thus, fruits and juices contain a significant amount of organic acids that cause a sharp decline pH of oral fluid (up to 4-3 units). If such a food product does not remain in the mouth for long, this change is short-lived. Longer contact can cause, for example, erosion of hard dental tissues: enamel and dentin. Some foods contain ammonium ions, urea (cheese, nuts, menthol) and are alcogenic. Typically, changes in the reaction of mixed saliva towards the alkaline side are insignificant and do not exceed pH 8.
Secondly, carbohydrates contained in food are metabolized by the microflora of dental plaque, with the formation of large amounts of organic acids, mainly lactate. The most acidogenic are mono- and disaccharides.
In descending order of acidogenicity, they can be arranged as follows: sucrose, invert sugar, glucose, fructose, maltose, galactose, lactose. The particular acidogenicity of sucrose is due to the adaptability of microorganisms to excess sucrose and is explained by its very rapid fermentation in dental plaque, a pronounced stimulating effect on the growth of dental plaque, and a high ability to stimulate the production of polysaccharides in dental plaque, in particular, polysaccharides with adhesive properties.
Thirdly, eating food and chewing it stimulate salivation and, thereby, help level out the resulting pH shifts.
Saliva
The acid-base balance in the oral cavity depends on saliva. Saliva is the main factor in leveling pH shifts in oral cavity under physiological conditions. Its influence on this indicator is due to:
- mechanical cleaning from food debris; 1
- antimicrobial effect of lysozyme, cyanide anions, phagocytes, immunoglobulins and other components;
- the work of buffer systems: bicarbonate (provides about 80% of the buffer capacity of saliva), protein and phosphate.
The implementation of the pH-stabilizing properties of saliva significantly depends on the rate of its secretion and rheological properties (viscosity). Generally, the higher the rate of salivation and the lower the viscosity, the stronger thethe ability of saliva to resist changes in pH in the oral cavity. Muscle contractions associated with chewing, swallowing and speech contribute to the emptying of the salivary glands and the movement of saliva in the oral cavity, and therefore can be considered as a factor in stabilizing the acid-base balance.
Methods of artificial influence on the acid-base balance in the oral cavity
The mechanisms of self-regulation of acid-base balance do not always work effectively enough. Therefore, various ways of influencing the main elements of regulation are used.
The most effective way is to influence the oral microflora and its metabolic activity. This influence can be carried out in several ways:
- mechanical removal using hygiene products (flossing and
brushing tongue, brushing teeth); - use of antiseptics, fluorides;
- limiting the intake of easily metabolized carbohydrates into the oral cavity
Another way of influencing the acid-base balance in the oral cavity is by influencing the oral fluid, for example, increasing the rate of salivation. Increased salivation is promoted by tougher foods (due to muscle activity), chewing gum, and adding small amounts of acids to food, such as citric acid.
An increase in the rate of salivation leads to an acceleration of the mechanical cleansing of the teeth and oral cavity from food carbohydrate residues, deflated epithelium, and there is an increased entry into the oral cavity of new molecules of buffer systems and antimicrobial components of saliva.
Assessment of the effects of factors affecting the acid-base balance in the oral cavity
It is obvious that the pH of the oral fluid is an indicator that changes under the conditions of the organism’s existence. A method for an integral assessment of factors affecting the acid-base balance in the oral cavity was proposed in 1938 by the American scientist Stefan. Information about the duration, severity of acidotic changes after eating and the speed of their correction can be obtained Stefan curve.
Stefan curve
Stefan curve is a graph of temporary changes in the pH of oral fluid (microbial plaque) after eating food. At the same time, it is precisely this information that makes it possible to predict the risk of adverse consequences of acid-base imbalances, and, in particular, such as demineralization of enamel. Consider the Stefan curve in oral fluid after eating a piece of sugar. The curve was obtained using repeated measurements of the pH of the oral fluid: before consuming sugar, 15, 30, 45 and 60 minutes after consumption.
It can be seen that within about 15 minutes after taking sugar, the pH drops to minimum values (catacrota). Then the pH rises with the restoration of the original level after an hour from the moment of taking sugar (anacrotic). The drop in pH is due to the production of acids by microflora, the restoration of the original pH value is due to the action of acid-reducing factors in the oral cavity. The assessment of factors that disturb the acid-base balance and factors counter-directed to them is carried out using empirical and calculated indicators.
Clinical significance of the Stefan curve is that it allows you to assess the cariogenic situation in the oral cavity. When the pH decreases below 6.2, saliva is a demineralizing liquid, and when the pH is above 6.2, it is a remineralizing liquid. Therefore, a saliva pH value of 6.2 is called critical. Using the Stefan curve, it is possible to study the cariogenicity (according to acid production) of various food products and the effectiveness of antimicrobial agents(antiseptics, hygiene products).
A number of studies allow us to evaluate individual factors affecting the acid-base balance in the oral cavity. This type of research includes analysis of the number of certain types of acid-producing bacteria in the oral cavity, as well as determination of the buffer capacity of saliva. The buffering capacity of saliva can be determined by the so-called “dipped stick” technique. The technique involves dipping a stick coated with chemical indicators into the patient's mixed saliva. The resulting color is an indicator of the buffer capacity of saliva.
Saliva buffer capacity
Buffer capacity of saliva. This is the ability to neutralize acids and alkalis. It has been established that eating carbohydrate foods for a long time reduces, and eating high-protein foods increases the buffer capacity of saliva. The high buffering capacity of saliva is a factor that increases the resistance of teeth to caries.
Yaroslav Solomiychuk, dentist
Why is acid-base balance so important for dental health? The ideal pH level for the oral cavity is above 7. The higher the acidity, the more favorable the environment for the development of microorganisms. An acidic environment occurs, for example, after eating foods rich in carbohydrates. Therefore, after eating such products, it is necessary to either brush your teeth or rinse your mouth with water (to at least reduce the acid concentration), but, indeed, the notorious chewing gum may be the best solution in the middle of the day. The components included in the gum neutralize acid, thereby restoring the acid-base balance in the mouth.
How to eat properly so that the balance of dental health is always normal? First of all, limit your consumption of carbohydrates, especially simple ones: sugar, sweets, confectionery. Sugar is the main enemy of teeth. And it’s not the amount of sweets that is more harmful, but the number of carbohydrate meals (including sweets). It is less harmful for dental health to eat 10 candies in one sitting than to eat 1 candy 10 times a day. In any case, high-carbohydrate foods should be “snacked” with black bread, raw vegetables, and a piece of hard cheese.
Another enemy of teeth is citric acid. It is added to almost all carbonated drinks as a preservative and flavor enhancer. It softens the enamel, makes it loose and leads to tooth erosion. The best way out(if you can’t refuse these drinks that are not healthy at all) - drink them through a straw and then rinse your mouth plain water. You should not brush your teeth after drinking soda, because the brush can damage the softened enamel.
Products containing fluoride, calcium and vitamin D are good for teeth (calcium is necessary for absorption by the body).
The most calcium is in dairy products. Vitamin D is found, for example, in sea fish, however, it is also produced by the body itself under the influence of ultraviolet rays and during prolonged exposure to the open air.
Fluoride is found primarily in black and green tea, sea fish and wholemeal baked goods, as well as in mineral water.
And the most useful product for teeth it's cheese. 100 grams of Dutch cheese covers the daily calcium requirement of an adult. Cheese also creates a protective shell on the teeth and neutralizes acidity in the oral cavity, so it’s not for nothing that in the best cuisines of the world cheese is usually served after dessert.
Green tea also has a similar neutralizing effect. Not only is it a source of fluoride, but it also inhibits the development of bacteria in the mouth, especially after eating sweets. And, unlike black tea and coffee, it does not stain teeth.
Teeth also “love” fruits and vegetables: currants, lettuce, cauliflower, pears, celery, sprouted wheat, cherries, grapes and onions. Apples put stress on teeth and gums, cleanse teeth of food debris, and contain calcium. Green apples are healthier than red ones, and domestic ones are healthier than imported ones. Carrots, like other vegetables, put stress on the teeth and gums, improving the flow of blood and oxygen.
Carrots and carrot juice improve the structure of teeth and promote the healing of wounds in the mouth. Radishes and cabbage strengthen teeth (contain calcium, magnesium, phosphorus). At the same time, cabbage also helps treat periodontitis. Cucumbers contain calcium and phosphorus, and cucumber juice has anti-inflammatory effects. Pumpkin helps prevent tooth decay due to its high fluoride content. Pumpkin milk porridge is especially good for teeth. 500-600 grams of pumpkin can provide a person’s daily need for fluoride. The “correct” pumpkin should be whole and have rich yellow or orange flesh.
Apricots also contain calcium, magnesium and phosphorus. By the way, in dried apricots the content of these substances is several times higher. Gooseberries are an excellent remedy for caries prevention due to their high fluoride content and the optimal composition of other “anti-caries” microelements. Beets are rich in microelements; and a piece of raw beets applied to the tooth can temporarily relieve toothache. One of the healthiest dishes for teeth is the well-known salad of beets, nuts and prunes, seasoned with sour cream.
Maintaining acid-base balance in the oral cavity is a very complex and important process. The oral cavity is in direct contact with environment, is the beginning of the digestive tract, has a heterogeneous topography of the oral organs and areas that are difficult to access for the self-cleaning process.
Factors that destabilize the constancy of the pH of oral fluid include: eating, taking medications, bad habits(smoking), professional harmful factors, falling from external environment or excreted from the body in saliva, metabolic activity oral microflora, the presence of diseases of the teeth and soft tissues of the oral cavity. Therefore, pH shifts in the oral cavity are often observed.
Changes in CBS in the oral cavity are physiological and pathological.
Physiological deviations are usually caused by food intake, are temporary, quickly compensated, do not lead to disturbances in physiological processes and do not cause structural changes in the tissues of the oral cavity. Somatic diseases and diseases in the oral cavity can cause persistent pathological changes CBS leading to significant changes in the structure and function of oral tissues.
The pH of the oral fluid undergoes daily fluctuations - at least in the morning, and in the evening the pH rises. At night, the pH of oral fluid is lower than during the day. Along with daily fluctuations, age-related changes pH. With age, the pH of oral fluid decreases. There is a natural decrease in the pH of saliva during pregnancy.
Obligate and facultative factors take part in the regulation of the constancy of CBS in the oral cavity.: CBS of the oral cavity depends on general condition the body, the nature of nutrition, working conditions, the state of salivation, chewing activity, the nature and activity of the oral microflora, the presence of artificial dentures, the state of oral hygiene and others.
1) However, the main natural regulator of acid-base balance in the oral cavity is saliva. Saliva has pronounced buffering properties, which are provided by three buffer systems, included in its composition, are bicarbonate, protein and phosphate. 80% of the buffering capacity of saliva is provided by the bicarbonate buffer system. It should be noted that buffer systems of oral fluid contain 6 times more alkaline-reacting components than acidic ones, due to which the buffering properties of saliva more pronounced when exposed to acidic foods.
The buffering function of saliva is subject to significant fluctuations and is determined, first of all, by its composition and quantity, which, in turn, depend on the functional activity of the salivary glands, the speed and nature of salivation ( 5 ), chewing activity. Stimulated saliva secreted under the influence of irritation taste buds, chewing. Of the taste stimuli, the most intense stimulant is sour taste. That is why, in order to prevent significant and long-term decline pH in the oral cavity is advisable to add a small amount of weak food acids (citric, acetic) to food and drinks. Stimulated saliva is different from unstimulated by secretion rate, composition, in particular, bicarbonate content. The concentration of bicarbonates in unstimulated saliva is within 1 mmol/l, and in stimulated saliva it increases to 15 mmol/l. Consequently, its buffering properties and ability to neutralize acidic products are more pronounced.
Chewing stimulates salivation. Use chewing gum even without flavoring agents, it has a beneficial effect on the pH of the oral cavity due to stimulation of saliva secretion. Flavoring additives to them contribute to more active stimulation secretion. It has been noted that the pH of saliva increases with increasing rate of excretion. More high speed salivation during the day than at night is determined by the fact that the pH value of saliva is higher during the day than at night.
Disorders of salivation and salivary composition, observed in many diseases, are accompanied by a persistent change in the acid-base state and insufficiency of buffer systems in the oral cavity.
To the number important factors self-regulation of CBS in the oral cavity can be attributed to tooth enamel. Tooth enamel is a kind of buffer system involved in maintaining proper CBS, namely, in binding excess hydrogen ions appearing on the enamel surface. As is known, the main component of enamel is hydroxyapatite, the crystals of which are capable of ion exchange. Into the interior of a hydroxyapatite crystal Only a few ions can penetrate - these are ions that are part of the crystal, or those close to them in structure and properties. Hydrogen ions are among those that penetrate into crystals relatively easily. At sharp increase Due to the acid content in the oral cavity, calcium ions leave the enamel, and two hydrogen ions take their place. Thus, the enamel absorbs excess hydrogen ions.
Of the destabilizing factors of the acid-base state in the oral cavity, first of all, it is necessary to point out food. Food products, depending on their nature, can change the pH of the oral cavity, both in the acidic and alkaline directions. However, if food products do not stay in the oral cavity for long, then these changes are insignificant and are quickly compensated.
The most pronounced pH shift in the oral cavity is observed after eating food containing simple carbohydrates - sucrose, glucose, fructose. It is even customary to talk about the specific effect of carbohydrates in the oral cavity, since similar changes are not observed when taking other foods. Simple carbohydrates undergo rapid fermentation by dental plaque microflora, causing a sharp activation of glycolysis, as a result of which organic acids - lactic, pyruvic, etc. - are formed and accumulated in the oral cavity. Their amount in the oral fluid increases 9-16 times within the next 20 minutes after taking sugar, which leads to a decrease in salivary pH. In addition, foods rich in sugars stimulate the growth of dental plaque (plaque) due to the fact that it promotes the proliferation of microorganisms, especially acid-forming ones. The latter are capable of synthesizing extracellular polysaccharides from sucrose - dextran, glycan and levan. Thanks to these reserves of polysaccharides in dental plaque (plaque), the formation of acids is possible for a long time after ingestion of carbohydrates.
At the surface of the enamel, the concentration of acids can be several times higher than in the outer layer of plaque.
Thus, the constant consumption of large amounts of carbohydrates leads to a shift in the pH of the oral fluid to the acidic side, which, according to modern ideas, contributes to the development of caries. However, it is shown that Cariogenicity of carbohydrates decreases with intensive chewing– acids formed when consuming carbohydrates are partially neutralized due to copious discharge saliva.
If the intake of carbohydrates is accompanied by the formation of acidic foods, then the intake of nitrogen-containing foods, which become a source of easily digestible nutritional substrates for oral microorganisms, leads to the accumulation of alkaline substances. As a result of metabolic transformations of amino acids and urea in the oral cavity, substances such as ammonia, mono- and diamines are formed, which are able to neutralize acidic foods and shift the pH of the oral fluid to the alkaline side. It is believed that the most important source of alkaline products in the oral cavity is the hydrolysis of urea by microbial urease to form ammonia and ammonium salts.
Consequently, two oppositely directed processes take place in the oral cavity: the accumulation of acidic products as a result of the fermentation of carbohydrates and the accumulation of alkaline products as a consequence of the utilization of nitrogen-containing substances. These two processes to a certain extent determine the pH of the oral fluid.
A significant factor influencing the CBS of the oral cavity is plaque. Dental plaque (plaque) is an accumulation of colonies of microorganisms of various types, mainly acid-forming, on the surface of the teeth. In addition to microorganisms, dental plaque includes a small amount of desquamated mucosal epithelium, salivary glycoproteins, and extracellular polysaccharides ( dextran, levan, glycan). Carbohydrates easily settle in dental plaque and contribute to its further formation.
Plaque accumulates most quickly in the interdental spaces and proximal surfaces of the upper chewing teeth, that is, in places where teeth cleaning is difficult.
Plaque microorganisms, utilizing food carbohydrates, produce large amounts of organic acids. The rate of formation of acids in dental plaque is very high and depends on many factors - the number and type of microbial populations of dental plaque, its substrate, localization and diffusion properties, the buffer capacity of saliva and the dental plaque (plaque) itself. Due to the low permeability of dental plaque, the resulting acids, on the one hand, are not able to diffuse beyond the plaque, and on the other hand, they are protected from the action of buffer systems. As a result, the concentration of hydrogen ions in dental plaque increases sharply; The pH on the enamel surface covered with dental plaque can drop to 4.5-5.0. This creates favorable conditions for focal demineralization of enamel and the development of caries. In addition to dental plaque, plaque on the tongue has a pronounced effect on CBS in the oral cavity.
The change in the pH of dental plaque or the oral fluid washing it, which develops after a sucrose load, is called Stefan curve.
In 1940, an American Robert Stefan(R. Staphan) after rinsing the mouth with solutions of glucose and sucrose, observed a rapid decrease in pH in dental plaque ( in 2-5 minutes) often to a level at which demineralization of the enamel occurs, followed by a slow return of pH to original level (in 30-60 minutes). Study of the Stefan Curve(its shape, amplitude, duration of recovery) has acquired practical significance. By the rate of pH decrease on the Stefan curve, one can judge the mass of dental plaque, its bacterial composition, the activity of enzymes produced by dental plaque microbes, the buffer capacity of dental plaque and oral fluid, which is necessary to predict susceptibility to caries, to assess the effectiveness of dental treatment. dental treatment. Thus, in patients with active caries or a high predisposition to it, the decrease in pH on the Stefan curve occurs quickly and to very low numbers. In this case, a slow recovery of pH to the original level is observed.
The Stefan curve is a highly informative test in assessing the cariogenic potential of chewing gums, food products, especially carbohydrate-containing ones (lollipops, chocolates, etc.), drinks containing sucrose (Fanta, Pepsi-Cola, etc.), and the anti-cariogenic properties of hygiene products.
Another factor that actively influences the CBS of the oral cavity is the metabolic activity of microorganisms. With pathology of the oral cavity and even in the presence of dentures, the composition of the oral microflora may change. By the way, dentures can significantly change not only the ecology, but also the ratio of factors regulating CBS in the oral cavity. The production of acids by microflora in the oral cavity is mainly anaerobic, and the main acid produced by bacteria is lactic acid. With a predominance of acid-forming microorganisms, capable of fermenting carbohydrates, the pH of the oral fluid deviates to the acidic side. When predominance of microbes producing urease, as is often the case with periodontal diseases, conditions are created for the pH to shift to the alkaline side.
Changes in CBS in the oral cavity can occur in the direction of both acidosis and alkalosis.
Oral fluid (mixed saliva) under physiological conditions is structured colloidal system and is a supersaturated solution of hydroxyapatite, or rather, the products of its hydrolysis - calcium ions (Ca 2+) and hydrogen phosphate (HPO 4 2–). It is believed that these components are part of colloidal micelles of calcium phosphate, which ensures their stability in a supersaturated state. Due to the oversaturation of saliva with these substances, an obstacle is created to the dissolution of tooth enamel, the introduction of calcium and phosphate ions from saliva into the enamel is facilitated, that is, in other words, the mineralizing function of saliva is carried out.
When the CBS shifts to the acidic side the stability of micelles and the degree of saturation of enamel with hydroxyapatite decrease. At the same time, they highlight two types of acid-base disorders in the oral cavity (V.K. Leontiev, 1978). The first type occurs at a saliva pH of 6.76-6.3. Saliva begins to lose its saturation with hydroxyapatite. In the composition of micelles, instead of basic phosphate (HPO 4 2–), acidic phosphate predominates - it does not participate in mineralization. Ca ions do not bind to the enamel matrix, so enamel decalcification prevails over mineralization.
Second type of CBS violation, according to V. Leontiev, it is observed when the pH of saliva decreases below 6.2-6.0. This pH value is considered critical when there is a sharp decrease in the saturation of saliva with hydroxyapatite. Saliva goes from a state of supersaturation to an unsaturated state, and from a mineralizing liquid it becomes a demineralizing liquid. The process of enamel mineralization stops completely, and the rate of enamel dissolution increases. When the oral fluid is acidified, the activity of proteinases increases, which also contributes to the demineralization of teeth.
In a neutral environment, mica evenly envelops the teeth, forming a special organic shell on them. The acidic environment promotes the precipitation of mucin, which begins to be deposited on the surface of the teeth. Loss of mucin contributes to the formation of dental plaque.
When the acid-base state shifts to the alkaline side the content of phosphates in the oral fluid increases, and poorly soluble calcium phosphate compounds Ca 3 (PO 4) 2 are formed, which leads to disruption of the micellization process. A large oversaturation of saliva with hydroxyapatite and disruption of the micelle formation process in an alkaline environment contribute to the formation of crystals and tartar. There is a point of view that the alkalization of oral fluid, which often occurs with gingivitis and periodontitis, is protective and compensatory in nature and is aimed at reducing the pathogenic effect of acids formed during inflammation. Be that as it may, but exactly alkaline environment contributes to the intensification of the process of plaque formation and the deposition of tartar in these diseases.
The most powerful factor that reduces the pH of saliva is microflora. A decrease in pH occurs when carbohydrates enter the oral cavity.
We studied the nature of salivation in confectioners. It was found that the content of residual sugars in the mixed saliva of confectioners in the middle of the working day exceeded the initial level by 5-7 times. Carbohydrates are an excellent nutrient medium for microflora.
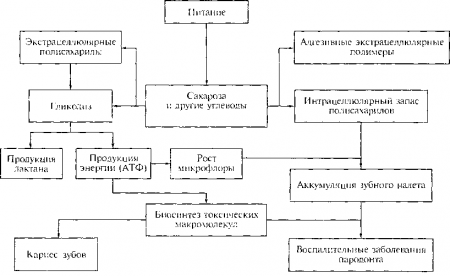
FEATURES OF CARBOHYDRATE METABOLISM IN THE ORAL CAVITY
The specific effect of carbohydrates on metabolism is due to the fact that they can enter into metabolic processes immediately in the oral cavity, where the conditions for the absorption of carbohydrates by microflora are close to ideal: here constant temperature, (~37ºС), moisture, close to neutral pH value.
Sugar (sucrose) and some other simple carbohydrates ( glucose, fructose) have a specific effect on the composition of saliva and metabolism in the oral cavity. It manifests itself in the fact that after taking simple carbohydrates, a kind of “explosion” occurs in the oral cavity. metabolic processes. The metabolic explosion is carried out by the microflora of the oral cavity and dental plaque. The conditions for the absorption of carbohydrates by microbes in the oral cavity are ideal.
Microbes very actively use carbohydrates for their needs and store them for future use in the form of reserve polysaccharides dextrans. Main recycling mechanism:
1. Happening significant activation of glycolysis and accumulation of lactic, pyruvic and other acids in the oral cavity. Their amount in saliva increases 9-16 times in the next 20 minutes after taking sugar, then quickly decreases, returning to the original level after 60-90 minutes.
2. This leads to acidification of saliva
3. the demineralizing effect of acids formed during glycolysis leads to leaching of calcium and increasing its concentration in saliva
4. At the same time, phosphorus is consumed for phosphorylation in energy processes, which leads to decrease in phosphate concentration.
Mechanisms of pathogenic effects of carbohydrates in the oral cavity
Metabolism of carbohydrates occurs in saliva and some other structures of the oral cavity. Even under physiological conditions, there are many organic acids in the oral cavity: lactic, pyruvic, acetic, and various amino acids.
Cariogenic processes occur most intensively in soft MN. The intake of easily digestible carbohydrates is the starting link in the chain of glycolysis reactions, which lead to disruption of the homeostasis of the oral cavity, the predominance of processes demineralization enamels.
Carbohydrate metabolism ends formation of organic acids, the increased concentration of which contributes to a local pH shift (in dental plaque) and the development of caries. In patients with caries, acid production is significantly higher, and normalization occurs much more slowly.
On the other hand, synthesized by microbes reserve polysaccharides – dextrans promote very tight attachment of microorganisms, and at the same time food debris, and all dental plaque to the surface of the enamel. All this also increases the risk of caries formation.
Research has shown that excess sugars in food lead to accumulation of glycogen V hard tissues teeth. The breakdown of enamel glycogen is considered one of the initial moments development of superficial carious lesions.
In addition, under these conditions it is easier to tartar formation, which subsequently leads to the development periodontal diseases.
The cariogenic role of carbohydrates depends not only on the consumption of large quantities, but also from reception frequencies sugar and its amount remaining in the mouth, physical properties sweet products(viscosity, stickiness). The more often and longer sugar stays in the oral cavity and comes into contact with the teeth, the more pronounced cariogenic effect it has
Longest Sugar-containing foods with a sticky consistency, such as chocolate, caramel, sugar syrup, etc., are retained in the oral cavity.
Long time
Carbohydrates are retained in the oral cavity when taking soft sweets with a higher concentration of sugar.
Doesn't last long in the oral cavity carbohydrates after drinking drinks with a sugar concentration of less than 10%.
On average, the highest glucose content in mixed saliva after eating sweets remains in the first 30 minutes.
Rinsing your mouth with tea soda solution or brushing your teeth can significantly reduce the concentration of glucose and its metabolites (pyruvate, lactate, etc.) in mixed human saliva after eating sweets.
Hygiene products significantly (almost 6 times) accelerate the elimination of carbohydrates from the oral cavity: 1-2% sodium bicarbonate solution, brushing with a toothbrush.
IMMUNOGLOBULIN A. IgA is the main class of antibodies in the oral cavity.
n Let's repeat: In blood serum IgA is contained in the form of monomers, dimers and tetramers, does not bind complement, and does not pass through the placenta.
In the blood, IgA makes up 20% of all Ig, concentration 2 g/l.
In saliva – mainly dimers, that is, IgA is not only in the blood, but is also secretory immunoglobulin. IgA is found in mucosal secretions (saliva, tear fluid, colostrum, bronchial secretions).
FIGURE: Polyglobulin Fc receptors on the basolateral surface of epithelial cells bind IgA dimer produced by plasma cells(finally differentiated B lymphocytes) into the extracellular space of the salivary gland. Together with this receptor, IgA penetrates into epithelial cells, but during the process of transcytosis (passing through cells), the receptor undergoes partial proteolysis, therefore, a complex of IgA dimer with a fragment of the Fc receptor (secretory form of IgA - sIgA) is secreted through the apical surface. Therefore, attached to IgA secretory component (SC) - a special protein synthesized by epithelial cells of the salivary glands. Complex sIgA molecules reach the surface of the epithelium and play a decisive role in the local immunity of the oral mucosa.
Transcytosis of IgA through epithelial cells into the glandular duct
n I.E. The biological role of this immunoglobulin is mainly local protection of the mucous membranes from infection. Immunoglobulins of this class bind to microorganisms and prevent their attachment (adhesion = adhesion) to the surface of epithelial cells, making reproduction difficult.
In addition to locally synthesized secretory IgA, the oral cavity also contains serum IgA that penetrates from the blood. Secretory IgA is more resistant to the action of proteolytic enzymes and is able to more effectively neutralize viruses, bacterial toxins, enzymes and agglutinate bacteria compared to serum IgA. The high resistance of sIgA to the action of proteolytic enzymes allows them to express their biological activity in environments with a high content of proteolytic enzymes, even in inflammatory exudates.
Class A immunoglobulins interfere with attachment wide range microorganisms to the mucous membrane and surface of the tooth, including cariogenic streptococcus (Str. mutans), which prevents the development of caries; act as opsonins and activate phagocytosis; neutralize viruses and prevent the absorption of antigens through the mucous membrane. The higher the sIg A, the higher the resistance to pathogens of bacterial, viral and fungal nature. Normal level synthesis of sIgA is one of the conditions for sufficient resistance of children in the first months of life to infections affecting the oral mucosa. IgA binds various antigens (food, microbial) and prevents sensitization of the body.
In addition to IgA, the oral cavity contains IgM and IgG. Their quantity is significantly lower (especially IgM) than IgA, but greater than with simple diffusion from the blood plasma, which indicates their partially local origin. Trace amounts of IgE were detected, which mainly enter the oral cavity from blood plasma like IgG - by passive diffusion.
The acid-base status in the oral cavity is an important component of local homeostasis. It provides many biochemical processes, such as re- and demineralization of tooth enamel, plaque and stone formation, vital activity of oral microflora, etc. The physical and biochemical properties of saliva, its mineralizing function, the activity of salivary enzymes, the transport of water and ions, the migration of cellular elements, the severity of cellular and humoral protective factors, the gradient and rate of ion exchange processes are closely related to the state of CBS in the oral cavity.
Therefore, violations of CBS lead to shifts in the homeostatic regulation of organs and tissues of the dental system. All changes in CBS in the oral cavity go in two opposite directions: towards acidosis or towards alkalosis. There are many factors that destabilize CBS in the oral cavity. These include food, water, air composition, meteorological and occupational factors, smoking and other bad habits, hygiene products, medications And therapeutic effects, finally, fillings and dental prostheses. With the progress of civilization, the number of such factors does not decrease, but increases. The oral cavity is a unique morphologically and functionally limited ecologically open biosystem.
Fluids, tissues, organs and anatomical formations. In Fig. Figure 10.4 shows a diagram of the main interactions in the CBS regulation system, from which it can be seen that the main liquid in the oral cavity, which implements ion exchange reactions between different zones, tissues and organs, is oral fluid, or mixed saliva. Gingival fluid is added to it, released from the gingival groove.
Basic mechanisms for regulating the acid-base state in the oral cavity.
Salivais the main fluid of the oral cavity, in addition, gingival and tissue fluid is constantly secreted here, diffusing through the mucous membrane.
The secretion of saliva in the glands goes through two stages. First, a primary isotonic secretion is formed in the acini of the salivary glands, the composition and properties of which are determined by passive ion transport and the action of electrophysiological mechanisms. Then, in the ducts of the glands, control and correction of the primary secretion is carried out depending on its composition and physiological need. This affects the acid-base properties of secreted saliva (Fig. 10.5).
Rice. 10.4. Scheme of the main interactions in the system of regulation of the acid-base state of the oral cavity

Salivary gland secretion pH 7.2
Rice. 10.5. The ion transport system in the tubules of the salivary glands, affecting the acid-base composition of saliva. ICP - interstitial duct cells
Interstitial cells of the duct are involved in the formation of the blood-salivar barrier, first described by Yu.A. Petrovich, which has high selectivity to ions. Excess hydrogen ions along with sodium ions from the gland duct enter the blood through passive reabsorption, which leads to a decrease in the acidity of saliva. And HCO3 ions from blood serum and tissue fluid selectively enter saliva by active transport, increasing its alkalinity. Due to this mechanism of regulation, the pH of secreted saliva can differ noticeably (by tenths of pH) from the always stable blood pH of 7.4. Mixed saliva is the main regulator of CBS in the oral cavity. The implementation of the functions of saliva significantly depends on the rate of its secretion, the amount in the oral cavity and rheological properties (viscosity, surface tension).
Interaction between microbial plaque and oral fluid.
Interactions occurring in the “dental plaque – oral fluid” system are the most frequent, rapid and pronounced. Microbial plaque is a strong factor in the destabilization of CBS in oral fluid. A change in the CBS in the oral fluid can occur either in the direction of acidosis or alkalosis (Fig. 10.6). Acidosis develops in dental plaque extremely quickly due to the predominance of acidogenic microflora, mainly streptococci, which ferment simple carbohydrates. Therefore, from the first minutes of use sweet food the concentration of hydrogen ions in dental plaque increases like an avalanche.

Rice. 10.6. Scheme of the main interactions in the “dental plaque - oral fluid” system in typical CBS disorders
The same buffer systems operate in the thickness of dental plaque as in saliva. However, due to the low diffuse properties of plaque, their effect is practically reduced to zero. Acids are washed away by oral fluid, the reaction of which (taking into account the buffer properties) changes in the acidic direction. The demineralizing properties of mixed saliva increase, and at pH below critical ( 6,2 - 6 , 0 ) it completely loses its mineralizing properties. At the same time, the microflora from saliva takes hydrogen phosphate ions, which they use in phosphorylation reactions that require energy.
Prolonged or frequently repeated acidosis on the surface of tooth enamel leads to its demineralization and the development of caries. This process is most likely in places where acidogenic microflora constantly accumulates (fissures and pits, cervical area and contact surfaces of teeth). In this case, tooth enamel begins to act as a kind of buffer system, taking part in the binding of hydrogen ions and, consequently, in reducing acidosis in the oral cavity. Therefore, the high activity of the carious process can be considered as the result of long-term decompensation of adaptive reactions aimed at combating acidosis in the oral cavity.
Alkalosis in dental plaque and oral fluid does not develop as quickly as acidosis, but nevertheless, changes in the reaction towards the alkaline side can be very pronounced. The main source of bases in dental plaque and oral fluid is urea. Some microorganisms of dental and lingual plaque (mainly periodontopathogenic) utilize urea, which is a substrate for the formation of ammonia using the enzyme urease. The conversion of accumulated ammonia into ammonium cation is the cause of alkalosis. Urea can enter the oral fluid in several ways; with food, secretions of the salivary glands (nitrates and nitrites), with gingival fluid, with blood plasma in case of bleeding of the gums and mucous membrane, as well as from decayed tissues. Urea can also be synthesized by microflora from amino acids contained in gingival fluid, dental plaque and mixed saliva ( L-arginine).
An important result of alkalosis in oral fluid and dental plaque is its mineralization, leading to the formation of tartar, which is also facilitated by an increase in the secretion of gingival fluid. It occurs in more than 80% of people. The process of stone formation under conditions of alkalosis is accompanied by an increase in the concentration of electrolytes in the oral fluid (Ca 2+, HPO 4 2-, Cl –, K 4, Mg 2+ ions, etc.), insufficient synthesis of protective proteins and disruption of their structure. Tartar becomes an additional buffer system in the oral cavity, formed under conditions of prolonged decompensation of the body's adaptive reactions aimed at combating alkalosis. Tartar formation reduces alkalosis in the oral cavity by binding hydrogen phosphate ions and hydroxyl ions.
Thus, decompensated disorders in the system of interaction “dental plaque - oral fluid” are important reason development of the most common dental and periodontal diseases. Demineralization of enamel in case of acidosis leads to the development of dental caries. Stone formation in the case of alkalosis, along with other factors (largely also dependent on local alkalosis), contributes to the aggravation of inflammatory reaction in periodontal tissues.
In addition to dental plaque, plaque on the tongue has a pronounced effect on CBS in the oral cavity. Its microflora, including a large proportion anaerobic microorganisms, takes part in the formation of dental plaque, as well as acids and bases in mixed saliva, and has a suppressive effect on acidogenic microflora. Muscular system maxillofacial area and oral cavity is an important factor in the regulation of CBS. Chewing, motility of the lips and cheeks contribute to more intense salivation, active excursion of oral fluid, and removal of food debris. In this regard, language plays a special role. It not only participates in the formation of the food bolus and self-cleaning of the oral cavity. The tip of the tongue is a mechanical regulator of the CBS, especially in the area of the oral and occlusal surfaces of the teeth. Being one of the “cleanest” areas in the oral cavity, almost devoid of microbial plaque, the tip of the tongue distributes saliva in the mouth, moves it and thereby accelerates ion exchange processes. Muscle contractions associated with chewing, swallowing and speaking help empty the salivary glands.
Methods for assessing the acid-base status in the oral cavity.
The assessment of the CBS in the oral cavity is given to the dentist useful information For early diagnosis, forecasting, monitoring treatment and prevention of major dental diseases. It allows you to choose methods pathogenetic treatment, carry out competent and adequate correction of nutrition, habits, hygiene, and, if necessary, plan orthopedic and orthodontic treatment, surgical interventions.
Various indicators can be used to assess CBS in the oral cavity. The potentiometric method is accurate, fast and affordable, for which laboratory pH meters with dial or digital display are used, equipped with a measuring electrode sensitive to hydrogen ions and an auxiliary reference electrode with a stable electrical potential.
Determination of the pH of saliva or a suspension of microbial plaque is carried out using standard glass electrodes. In this case, the liquid to be tested is placed in a small cuvette. To determine pH directly in the mouth, metal oxide measuring electrodes made of antimony or special olives in which the measuring and reference electrodes are sealed are more convenient. There is a radiometric method for determining pH in the mouth (from a distance).
The pH value of oral fluid in the same individuals without any stimulation is constant. During the day, regular temporary fluctuations in the pH of saliva occur: in the morning it is lower than in the middle of the day, and tends to increase in the evening. At night, the pH of mixed saliva is lower than during the day. Along with the daily rhythm of changes in the pH of the oral fluid, a decrease in its values with age was noted. A decrease in pH is observed in women during pregnancy. In different parts of the oral cavity, the pH value is different: on the mucous membrane hard palate reaction by 0.7-1.2 units. more alkaline than in other areas; in the area of the lower lip it is 0.3 -0.8 units. more alkaline than in the upper region.
In 1940, the American dentist R. Stefan, after applying solutions of glucose and sucrose to the teeth, observed a rapid decrease in pH in dental plaque, followed by a slower return to the original level. This change in pH of plaque or mixed saliva as a result of microbial glycolysis of sugars is called the Stefan curve (Fig. 10.7). V. A. Rumyantsev identifies in this curve the following informative calculated indicators: amplitude of the Stefan pH curve
catacrotic slope
anacrotic slope
asymmetry coefficient
![]()
intensity of critical pH decrease
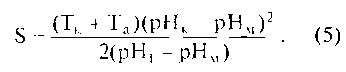
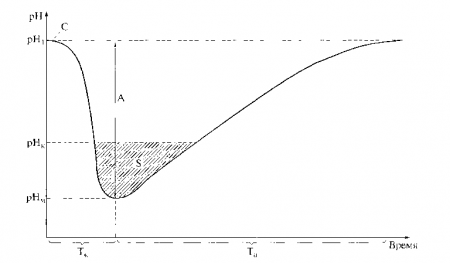
Rice. 10.7. Curve (Stefan curve) of changes in the pH of mixed saliva after consuming sucrose (C): pH1 - initial pH value; A is the amplitude of the curve; Tk - duration of catacrota; Ta - duration of anacrota; rnk - critical pH value; S - intensity critical value pH; pHm - minimum pH value
The amplitude of the curve is the most informative indicator, since it characterizes the acid-producing activity of the oral microflora and the effectiveness of the mechanisms regulating CBS. The greater the amplitude of the curve, the more organic acids (mainly lactate) are produced in response to carbohydrate stimulation of the microflora and the less ability the CBS regulation systems have to eliminate acidosis. The value of the catacrotic coefficient increases with the increase in the rate of microbial acid production and, to a greater extent than the amplitude, characterizes its acidogenic activity. The anacrotic coefficient, on the contrary, indicates the ability of the CBS regulation systems to restore homeostasis.
Using the asymmetry coefficient, one can judge the degree of destabilizing effect of carbohydrate-containing products on WWTP. The intensity of the critical decrease in pH characterizes the severity of exorbitant changes in the CBS, which can lead to the development of pathology (demineralization of hard dental tissues). The listed indicators of the Stefan curve reflect short-term disturbances CBS in the oral cavity. J. Nikifruk provides data that the daily intensity of the critical decrease in pH in dental plaque is several times greater in caries-susceptible individuals compared to caries-resistant individuals.
The use of a test carbohydrate-containing product (identical in composition, concentration and time of application) as a stimulator of acidogenic oral microflora made it possible to use the Stefan curve to assess the inhibitory effect on microflora various means. Comparison of the amplitudes of pH test curves in oral fluid before and after the use of antimicrobial agents allows one to assess the degree and duration of their suppressive effect, as well as compare the effectiveness of different concentrations, fillers (solvents), and duration of use. The method also turned out to be useful in assessing the effectiveness of oral hygiene products and the effect of food products on CBS in the mouth.
pH value and food products.
Acidic foods and drinks (fruits, juices, etc.) cause a sharp change in the pH of saliva in the acidic direction: below 5.0. If food does not stay in the mouth for long, these changes are short-term and are quickly compensated by the buffer systems of the released saliva. Longer presence of such products in the mouth can have a destructive effect, for example, cause erosion of hard dental tissues. Drinks containing sucrose (Coca-Cola, Pepsi-Cola, Fanta, lemonade, sweet carbonated drinks) significantly reduce the pH of dental plaque.
The most acidogenic in foods are di- and monosaccharides. Among them, sucrose comes first. Its special acidogenicity and cariogenicity is explained by its very rapid fermentation in dental plaque and its high ability to stimulate the production of extracellular polysaccharides (Fig. 10 . 8 ).
Sugars can be arranged in descending order of specific acid-producing potential as follows:
- sucrose;
- invert sugar;
- glucose;
- fructose;
- maltose;
- galactose;
- lactose.
The duration and severity of the decrease in pH after eating carbohydrate foods is largely determined by such characteristics as the time spent in the oral cavity, the concentration of sugars in the product, the composition and amount of oral microflora, the rate of salivation and ingestion of the product and saliva, and the frequency of food intake. Already 30 s after eating a carbohydrate food, the sugar concentration in mixed saliva increases sharply and then decreases. The decrease in concentrations occurs mainly due to the adsorption of sugars in the composition of microbial polysaccharides. A significant role in the retention of carbohydrates in the mouth is played by the process of self-cleaning (saliva, tongue). The most pronounced acidogenic potential is found in foods such as sugar, chocolate, sweet dough products, muffins, bread, chocolates, cakes, caramel, and ice cream. Cow's and mother's milk have low acidogenicity compared to sugars.
Along with food products, causing acidosis in the oral cavity, there are many products that change the EOS to the alkaline side, these include nuts, cheese (especially Cheddar varieties), and menthol. This effect is explained by the presence in them of ammonium-containing substances, urea and substances that, upon dissociation, form ions that actively bind hydrogen ions, as a result of which the pH of saliva increases by 0.5 - 0.7.
Control questions
- What types of CBS pathology do you know?
- Name the main buffer systems.
- What indicators are used in diagnosing CBS disorders?
- What are compensated and decompensated forms of CBS impairment?
- Name the reasons for the development of respiratory acidosis. What compensatory mechanisms are formed in this form of CBS pathology?
- Name the reasons for the development of metabolic acidosis. What compensatory mechanisms are formed in this form of CBS pathology?
- Name the reasons for the development of respiratory alkalosis. What compensatory mechanisms are formed in this form of CBS pathology?
- Name the reasons for the development of metabolic alkalosis. What compensatory mechanisms are formed in this form of CBS pathology?
- How do blood counts change during different forms CBS violations?
- Name the main forms of impairment of CBS in the oral cavity.
- Give the main mechanisms of pH shifts in the oral cavity.
- What are the principles for diagnosing impairment of CBS in the oral cavity?
If you find an error, please highlight a piece of text and click Ctrl+Enter.








