Not all diseases of cardio-vascular system today they threaten premature death, some of them are quite easy to get rid of. However, there are deviations that are genetic in nature. This includes Brugada syndrome. This disease is dangerous not because of changes in the electrocardiogram, but because it significantly increases the risk of sudden cardiac death.
This syndrome was discovered by two Spanish cardiologists in 1992, and one could read about it in scientific reference books starting in the late nineties. However, if we consider the practical side of this issue, we have to admit that today few doctors have information about this disease, and therefore cannot identify it and begin appropriate treatment.
Josep Brugada and his brother Pedro discovered and described Brugada Syndrome
The number of people affected by this disease is rising sharply in east and south Asia, where five out of every 10,000 people have it.
In Western countries, the ratio is lower - two people out of 10,000. It is also noted that this condition is more common in people in the age group of 30 to 40 years, and among them men are most often affected.
Causes
At first it was thought that the disease develops in those patients who have lesions of the coronary vessels. There was also an opinion that the syndrome could develop in those who have suffered a myocardial infarction and have a history of acquired or congenital pathologies blood vessels and heart. However, it was later found that sudden death can overtake those who are not associated with cardiac ailments. What are the causes of the syndrome?
This condition is based on inheritance along a dominant and autosomal path, more precisely, on the mutation of several genes responsible for the formation of the anomaly. It turns out that these mutations may be the cause of the development of the disease. At the same time, it has been confirmed that in many patients this pathology had no genetic confirmation.
It was concluded that the autonomic nervous system may actively participate in the development of the syndrome. It is believed that with inhibition and activation of the nervous parasympathetic system Arrhythmogenesis increases, so syncope attacks mainly occur at night or in the evening.
If, however, the cause lies in genetics, it is important to understand that the syndrome can develop due to anomalies occurring in the electrophysiological activity of the right cardiac ventricle at his exit. The mutated gene, which is located on the third chromosome, is involved in coding sodium channels, or more precisely, the structure of its protein. These channels provide potential action current Na.
 Comparison of PD in the wall of the right ventricle and ECG in normal conditions and in Brugada syndrome
Comparison of PD in the wall of the right ventricle and ECG in normal conditions and in Brugada syndrome It is calculated that there are at least 80 mutations occurring in the SCH 5A gene. They are observed in a quarter of patients and often within the same family. Of course, an important role is played in the formation of the disease. pathological changes, which occur in other genes and are responsible for encoding channels and proteins.
Despite all this, it should be recognized that the reasons are still unclear and cannot be clearly classified. Most conclusions are made after autopsy of people who died suddenly. These findings suggest that the risk sudden death rises in following cases:
- situational fainting;
- blockade of the His bundle, his right leg;
- specific signs detected on the ECG;
- early causeless attacks of sudden fainting, especially if tachycardia was observed at that moment;
- sudden death of direct relatives.
Symptoms
Brugada syndrome is characterized by two groups of main symptomatic signs:
- signs of sudden death;
- states of syncope.
Almost 80% of patients who suffered sudden cardiac death had a history of syncopal attacks. The most severe cases were accompanied by fainting combined with convulsions. However, attacks can occur without fainting states, but in this case other signs appear:
- sudden weakness;
- pallor;
- interruptions in heart function.
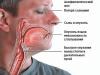
 This is a genetic disease characterized by changes in the electrocardiogram
This is a genetic disease characterized by changes in the electrocardiogram The main clinical signs are based on the development of ventricular tachycardia and fibrillation. They mainly manifest themselves as supraventricular tachyarrhythmias.
Periodic symptoms of ventricular arrhythmias are most common in men over 38 years of age, however, there have been cases when such a picture was observed in older people and children.
Brugada syndrome usually occurs during rest or sleep, especially when the heart rate is reduced. But one cannot ignore the fact that 15% of attacks of this pathological nature occurred after physical exertion.
Ventricular arrhythmia can occur after drinking alcohol. 93% of ventricular fibrillation occurred at night, 7% during the day. During wakefulness, the syndrome developed in 13%, and during sleep in 87%.
It turns out that the following main signs of the syndrome can be identified:
- VF episodes;
- nocturnal attacks accompanied by severe respiratory distress;
- ventricular tachycardia.
In addition, there are episodes of sudden death. These are conditions in which there is no electrical activity heart and breathing, but the person comes to his senses. This can happen by accident or due to timely seeking help. So, the syndrome is indicated by sudden fainting, rapid heartbeat and lack of air.
Diagnostics
Today the main and effective diagnostic method– ECG. It is with its help that you can determine the signs of right bundle branch block (RBBB).
At the same time, in some leads there is ST-segment elevation and characteristic symptoms pathology. Sometimes T-wave inversion is present.

Brugada syndrome can be identified on the ECG using two types of elevation:
- ST segment elevation in the form of a “saddle”
- elevation of the ST segment in the form of a “vault”.
There is a connection between this segment and ventricular rhythm disturbances. For example, if a patient has a second type of elevation, symptomatic forms of pathology will predominate, which will be indicated in the anamnesis in the form of syncopal attacks or ventricular fibrillation. In these patients, sudden death is diagnosed more often than in those patients who have the first type of segment elevation in combination with the asymptomatic variant.
Standard, transient ECG changes make diagnosis difficult, leading to reliance on less reliable methods to confirm the presence of the syndrome. In this regard, in order to confirm the diagnosis, high right chest leads can be used, recorded at the second or first intercostal spaces.
A study was conducted of patients who survived SCD, the causes of which were unknown, as well as their relatives. The results of the study showed that signs of pathology during a standard examination were identified in 70% of patients and 3% of relatives, but with additional examination indicators increase by 92% and 10% respectively.
Registration of ECG indicators in the case of administration of antiarrhythmic drugs, which include Aymalin, Flecainide and Procainamide, is considered quite promising. However, in this case, medical personnel must be very well prepared for the development of paroxysmal VF and TJ, because the risk of these conditions increases sharply in the case of this examination.
After taking antiarrhythmic drugs, there have been cases of description of a latent form of pathology. However, in this case, class C drugs were taken when class A was ineffective. To detect a hidden syndrome, the drug Dimenhydrinate is used.
Particular attention is paid to the febrile state. However, it is quite difficult to identify hidden forms today, because in clinical practice Genetic diagnostic methods are rarely used. Mutations that occur in genes are not immediately detected. In the syndrome, pathology is not determined by such research methods as coronary angiography, echocardiography and endomyocardial biopsy.
Treatment
It is worth recognizing that there is still no clear drug treatment. The fact is that there are no generally accepted drugs that reliably reduce the likelihood of sudden death. To date, drugs such as Propranolol and Disopyramide have been confirmed.
They are good at preventing cardiac rhythm disturbances. However, there are cases when the use of even these medicines led to ST segment elevation.

Sometimes intravenous administration of Isoproterenol is used, which can lead to the cessation of VF relapses. Some experts believe that taking amiodarone and beta blockers at the same time will not prevent SCD.
Brugada syndrome remains an incompletely understood condition, so a search is underway medical supplies, which may be effective in its treatment. A single case of taking Cilostazol has been described, which prevented regular episodes of VF. Adrenergic agonists, adrenergic blockers and catecholamines reduce the elevation of a characteristic segment.
You need to know that today there are the only and effective method treatment of those patients who have a symptomatic version of the syndrome - implantation of a cardioverter-defibrillator. It helps prevent episodes of sudden death.
If the action of this device is combined with the administration of amiodarone, it will be possible to reduce the frequency of its discharges. There are the following indications for implantation of this device:
- men aged 30 to 40 years;
- patients whose direct relatives died from SCD;
- spontaneous ECG changes;
- confirmed gene mutation.
Consequences
After all of the above, it becomes clear that Brugada syndrome has an unfavorable prognosis. Death occurs due to VF. Risk fatal outcome the same for periodic and constant changes in the ECG.
It is difficult to say anything specific regarding preventive measures, which can reduce the risk of sudden death, especially when it comes to genetic predisposition to the appearance of this disease. However, it is important to understand that healthy image life and a good mood will help you not to focus on your illnesses, and sometimes they can save your life.
Brugada syndrome is a rare hereditary disorder of the heart's electrical system that can lead to ventricular fibrillation in otherwise healthy young people. Unlike most other diseases that cause sudden death in young people, Brugada syndrome usually occurs during sleep rather than during activity or exercise.
The heart has its own electrical (conducting) system, consisting of an electrical impulse generator - the main pacemaker (sinus node) - and conducting pathways (atrioventricular junction, bundle of His and its branches) connecting the entire electrical circuit.
Most people diagnosed with Brugada syndrome are young, middle-aged adults. average age who are 41 years old at the time of diagnosis. Brugada syndrome is much more common in men than women—in some studies, the prevalence in men is nine times higher than in women.
Brugada syndrome is thought to affect about one in 10,000 people in the United States. However, it is more common in people of Southeast Asian descent (approximately 1 case in 100 people). The only cardiac abnormality is electrical; the hearts of people with Brugada syndrome are structurally normal.
Symptoms
The most devastating problem caused by Brugada syndrome is sudden death during sleep. However, people with Brugada syndrome may experience episodes dizziness, loss of balance or (loss of consciousness) to fatal outcome. If the first signs come to the doctor's attention before death, a diagnosis can be made and treatment can be prescribed to prevent subsequent sudden death.
Originally identified as a mysterious "unexpected/unexplained nocturnal death," Brugada syndrome was first described decades ago as a condition affecting young men in Southeast Asia. It has since been recognized that these young Asian men have Brugada syndrome, which is much more common in this part of the world than in most other places.
Causes and risk factors
Brugada syndrome appears to be caused by one or more genetic abnormalities that affect heart cells, particularly in the genes that control the sodium channel. It is inherited in an autosomal dominant manner, but not everyone who has the abnormal SCN5A gene or genes is affected equally.
The electrical signal that controls heart rhythm is generated by channels in the membranes of the heart cell that allow charged particles (called ions) to flow back and forth across the membrane. The flow of ions through these channels produces the heart's electrical signal. One of the most important channels is the sodium channel, which allows sodium to enter the heart cells. In Brugada syndrome, the sodium channel is partially blocked, so that the electrical signal generated by the heart is altered. This change leads to electrical instability, which under some circumstances can lead to ventricular fibrillation.
Additionally, people with Brugada syndrome may have a form of dysautonomia, an imbalance between sympathetic and parasympathetic tone. It has been suggested that the normal increase in parasympathetic tone during sleep may be exaggerated in people with Brugada syndrome, and that this strong parasympathetic tone may cause abnormal channel instability and lead to sudden death.
Other factors Activities that can cause fatal arrhythmias in people with Brugada syndrome include fever, cocaine use, and the use of various medications, especially some antidepressants.
Diagnostics
Electrical abnormalities caused by Brugada syndrome can result in the characteristic ECG pattern- a pattern called Brugada pattern. This pattern consists of a pseudo-right bundle branch block followed by ST segment elevations in leads V1 and V2.
(A) - normal ECG in the right chest leads (V1-V3); (B) - changes in Brugada syndrome
Not everyone with Brugada syndrome has the "typical" Brugada pattern on the ECG, although they are likely to have other subtle changes. Thus, if Brugada syndrome is suspected (eg, due to syncope or sudden death of a family member during sleep), any ECG abnormalities should be referred to an electrophysiology specialist to assess whether the person may have an "atypical" Brugada pattern.
If a person's ECG shows Brugada pattern, and if he had episodes of unexplained severe dizziness or fainting, the person has suffered cardiac arrest or there has been a history of sudden death in the family before the age of 45, the risk of sudden death is high. However, if the Brugada pattern is present on the ECG but none of the above signs and symptoms are present, the risk of sudden death is much lower.
People with Brugada syndrome who are at high risk of sudden death should be treated aggressively. However, for those who have a Brudada pattern on the ECG but no other risk factors, the decision about how aggressive treatment should be is not as clear-cut.
Electrophysiological study is used to help guide more aggressive treatment by clarifying a person's risk of sudden death. The ability of electrophysiological testing to accurately assess risk is less than ideal. However, major professional societies now support this study in people who have a Brugada pattern on ECG without additional risk factors.
Genetic testing may help confirm the diagnosis of Brugada syndrome, but usually does not help in assessing a patient's risk of sudden death. Besides, genetic testing for Brugada syndrome is quite complex and often does not provide clear answers. Therefore, most experts do not recommend routine genetic testing in people with this disease.
Since Brugada syndrome is genetic disease, which is often inherited, current recommendations require screening of all first-degree relatives of anyone diagnosed with the condition. Screening should consist of an ECG examination and a careful review of the medical history, looking for episodes of fainting or severe and frequent dizziness.
Treatment
The only proven method for preventing sudden death in Brugada syndrome is to install implantable defibrillator. In general, antiarrhythmic drugs should be avoided. Because of the way these drugs affect channels in the membranes of heart cells, not only do they not reduce the risk of ventricular fibrillation in Brugada syndrome, but they may actually increase this risk.
Whether someone with Brugada syndrome should have an implantable defibrillator depends on whether their risk of sudden death is definitively assessed as high or low. If the risk is high (based on symptoms or electrophysiological studies), a defibrillator is recommended. But implantable defibrillators are expensive and carry their own complications, so if the risks of sudden death are assessed as low, these devices are not currently recommended.
Whenever a young person is diagnosed with a heart condition that could lead to sudden cardiac arrest, the question must be asked whether various exercises are safe to perform. This is because most of the arrhythmias that lead to sudden death in young people are more likely to occur during exercise.
In Brugada syndrome, on the other hand, fatal arrhythmias are much more likely to occur during sleep than during exercise. However, it has been suggested (with virtually no objective evidence) that strenuous exercise may pose a higher than normal risk of cardiac arrest in people with the condition. For this reason, Brugada syndrome is included in formal guidelines developed by expert panels that address exercise recommendations for young athletes with heart disease.
Initially, guidelines for playing sports with Brugada syndrome were quite restrictive. The 2005 Bethesda 36th Conference on Guidelines for Athletes with Cardiovascular Impairment recommended that people with Brugada syndrome avoid high-intensity physical activity altogether.
However, this absolute restriction was later found to be too strict. Because the arrhythmias seen with Brugada syndrome do not typically occur during exercise, these recommendations were liberalized in 2015 under new guidelines from the American Heart Association and the American College of Cardiology.
- They, their doctors, parents or guardians understand possible risks and agreed to take the necessary precautions.
- An automatic external defibrillator (AED) is a normal part of their personal sports equipment.
- Team officials are able and willing to use an automated external defibrillator and perform CPR (cardiopulmonary resuscitation) if necessary.
To summarize
Brugada syndrome is a rare genetic disorder that causes sudden death, usually during sleep, in healthy young people. The importance is to diagnose this condition before an irreversible event occurs. This requires doctors to be vigilant - especially in those who have had fainting or unexplained episodes of dizziness - to subtle ECG data which are observed in Brugada syndrome.
People diagnosed with Brugada syndrome can almost always avoid death with appropriate treatment and can expect to live a normal life.
Interesting

Higher education(Cardiology). Cardiologist, therapist, doctor functional diagnostics. I am well versed in the diagnosis and treatment of diseases respiratory system, gastrointestinal tract and cardiovascular system. Graduated from the Academy (full-time), with extensive work experience behind her. Specialty: Cardiologist, Therapist, Functional Diagnostics Doctor. .
With elevation of the J point and ST segment in the right precordial leads and manifested clinically by recurrent syncope, as well as cases of sudden cardiac death, which occurs more often in males aged 30–40 years, was described by P. Brugada and J. Brugada in 1992 The disease is inherited in an autosomal dominant manner, and is characterized by incomplete penetrance of genetic changes.
Ventricular tachycardia, (mostly polymorphic, extremely rarely monomorphic) characterized by a high risk of transformation into ventricular fibrillation, is the main clinical manifestation of Brugada syndrome. They typically occur at rest, during night sleep (Fig. 1), which makes their detection more likely using a HM ECG rather than with a standard ECG recording. One of the clinical manifestations accompanying these arrhythmic events may be episodes of hoarse (agonal) breathing during sleep. Ventricular tachycardia can be provoked by feverish conditions, as well as a number of medications (see Table 1). Symptoms of the disease usually appear in adults, and the average age of onset of cases of sudden cardiac death is 41 ± 15 years. In addition, with Brugada syndrome, cases of supraventricular tachyarrhythmias are recorded more often than in the general population.
Rice. 1. Unstable paroxysm (highlighted by a frame) of polymorphic ventricular tachycardia (heart rate 160–180 beats/min.). Holter monitor ECG recording in 12 leads in a patient with Brugada syndrome. The time of onset of paroxysm is 23 hours. The arrows in lead V1 indicate the elevation of the J point during sinus rhythm contractions.
Epidemiology
The prevalence of the disease in the general population is currently unknown. It is much more common in the countries of Southeast Asia (Asia-Pacific region), where its prevalence reaches 0.5–1:1000. Brugada Syndrome (BrS) is detected in individuals who do not have signs of organic heart disease; in men it occurs 8–10 times more often than in women, which is presumably due to the greater strength of the short-term outgoing current of potassium ions Ito (one of the currents , involved in the formation of the syndrome) and the effect of higher concentrations of testosterone.Etiology
Brugada syndrome is caused by genetic mutations that lead to a decrease in the strength of incoming sodium (INa) and calcium (ICa,L) currents or an increase in the strength of outgoing potassium currents (Ito,f, IKs, IK,ATP).Classification
Currently, 12 genetic variants of the syndrome are known, they are presented in table 1. Molecular genetic methods make it possible to detect mutations in approximately 1/3 of patients with obvious clinical and instrumental manifestations of Brugada syndrome, which indicates the genetic heterogeneity of the disease and suggests the discovery of a large number of new, currently unknown mutations in the future. The most common mutations in the SCN5A gene are found in almost 30% of patients.Table 1. Molecular genetic types of Brugada syndrome

Diagnostics
The basis for diagnosing Brugada syndrome is the registration of ST segment changes on the ECG that are pathognomonic for this disease in the absence of structural heart disease and other conditions in which similar ECG changes can be recorded (listed below). Based on the nature of changes in the final part of the ventricular complex, three ECG types of the Brugada phenomenon are distinguished (Table 2, Fig. 2).Table 2. ECG types of Brugada phenomenon


ECG recording should also be carried out by placing the electrodes of the right precordial leads (V1–V2) above the standard position, up to the 2nd intercostal space. Detection of pathognomonic ECG changes in these positions has the same diagnostic significance as with standard electrode placement. Changes in the terminal part of the ventricular complex, characteristic of Brugada syndrome, may be transient. Therefore, in cases where the available ECG recordings do not contain signs that fully fit into the diagnostic criteria, but there is reason to assume the presence of Brugada syndrome, it is advisable to conduct diagnostic provocative drug tests using sodium channel blockers administered intravenously - ajmalina (at a dose of 1 mg/kg; not registered in Russia) or procainamide (at a dose of 10 mg/kg), allowing in some cases to “expose” the signs of this syndrome. Pharmacological challenge tests should be performed by experienced medical personnel when ECG monitoring patient and the mandatory possibility of organizing resuscitation measures in case of induction of life-threatening ventricular arrhythmias under the influence of administered drugs.
In accordance with the modified diagnostic criteria, to make a diagnosis of Brugada syndrome, registration on the ECG of spontaneous or drug-induced ST segment elevation of the “fornix” type (type 1) in at least one of the right precordial leads (V1–V2) is necessary when the electrodes are located in a typical place or installing them in the 2nd intercostal space.
Molecular genetic diagnostic methods are also important for diagnosing the disease, however, genetic mutations in patients with Brugada syndrome can be detected only in approximately 30% of cases, so a negative result of genetic analysis does not completely exclude the diagnosis of Brugada syndrome. If a patient has Brugada syndrome, genetic mutation Screening aimed at identifying this mutation is recommended for all close relatives, even if they do not have ECG changes characteristic of this disease. Conducting molecular genetic studies in persons with ECG changes of types 2 and 3, in the absence of clinical manifestations of Brugada syndrome and a family history of SCD, is currently not recommended.
Differential diagnosis
Brugada syndrome should be differentiated from others possible reasons syncope, given the relatively youngBrugada syndrome is a genetic abnormality that leads to irregular heartbeat rhythms. The exact prevalence of the disease is unknown. This is due to the difficulty of diagnosing the pathology, since the disease may not manifest itself clinically. Doctors suggest that Brugada syndrome occupies a leading position among the causes of sudden death in young patients. Treatment of the disease is based on both the use medications, and during the operation to install a defibrillator.
Causes and classification of Brugada syndrome
It is known that the pathology is hereditary in nature. According to the latest available information, there are at least 6 genes whose mutations provoke the occurrence of specific signs. Based on this differentiation, the small literature on Brugada syndrome describes several variants of the disease. The classification is as follows:
- The most common and well-studied type of pathology is BrS-1. Mutation of the SCN5A region, which is located on the arm of the third chromosome, leads to a change in the functioning of the type 5 sodium channel. This structure takes an active part in the process of transmitting nerve impulses in the heart muscle. It has been proven that gene changes lead to other conditions leading to cardiac diseases.
- Type BrS-2 is associated with a mutation in GPD1L, a structure that is responsible for the synthesis of peptides that catalyze various chemical reactions in the heart muscle. The occurrence of symptoms of Brugada syndrome is also associated with dysfunction of sodium-potassium channels.
- BrS-3 is a type of problem in which a mutation occurs on the 12th chromosome. The structure of the CACNA1C gene is transformed, which leads to a change in the normal transport of calcium in cardiomyocytes. Element plays important role in the conduction of nerve impulses, therefore, malfunctions of this structure lead to severe arrhythmia, and are also a common cause of sudden death in patients.
- In type BrS-4, a mutation of the CACNB2 gene, which is located on the 12th chromosome, is diagnosed. It also disrupts the natural functioning of calcium channels.
- BrS-5 is a common type of pathology, which is caused by changes in the structure of SCN4B. The gene is located on chromosome 11 and is responsible for the synthesis of a protein that ensures the transmission of nerve impulses in cardiomyocytes. This is possible due to the fact that the protein is part of small sodium channels.
- Type BrS-6 is associated with the SCN1B mutation. This type of Brugada syndrome is similar in clinical course and pathogenesis with the first. This feature is due to the fact that a section of DNA located on chromosome 19 ensures the functioning of type 5 sodium channels.
Main signs of pathology
Clinical picture diseases are often nonspecific. This fact greatly complicates the process of diagnosing the disease. In most cases, the signs of Brugada syndrome are limited to fainting, as well as attacks of rapid heartbeat at night. The literature also describes cases where the disease was an incidental finding in clinically healthy patients. For this reason, researchers associate many sudden deaths that occur as a result of disturbances in the rhythm of cardiac structures with this genetic disease. In the articles describing Brugada syndrome, which can be found in the UDC, only the criteria for making a diagnosis based on ECG results are described in detail. Therefore, symptoms of a lesion are often not used to confirm the presence of a problem. In addition to general weakness, syncope and attacks of tachycardia, in the absence of physical activity, patients also suffer from abnormal reactions to certain medications, for example, antihistamines and beta-blockers. Clinical signs of pathology are most often noted at the age of 30–40 years, however, in the literature there is also data on identifying the disease in children.
Diagnostic tests
Confirmation of the presence of a disease is an important issue in modern medicine. The difficulty in identifying the problem stems from the fact that it rarely manifests itself and only causes sudden death. In order to prevent such consequences of the occurrence of a genetic disease, diagnostic criteria have been developed that involve a detailed description of the results of the electrocardiogram for Brugada syndrome. This method is considered the main way to confirm the presence of the disease, since only with its help doctors can detect specific abnormalities in the functioning of the heart. When comparing ECG healthy person and a patient with a congenital disorder of nerve impulse transmission, the following symptoms are noted:
- The typical picture of the disease involves a rise in the ST complex, which characterizes the excitation coverage of both ventricles, above the isoelectric line. The T wave, reflecting the process of repolarization of these chambers of the heart, becomes negative.
- Brugada syndrome on the ECG is associated with the appearance of signs of complete or partial block of the bundle branches. These connections ensure the conduction of nerve impulses to the ventricles.
- Holter monitoring is considered informative for the disease. This method involves taking a 24-hour electrocardiogram and is widely used in cases of suspected rhythm disturbances. ECG in Brugada syndrome is characterized by the presence of attacks of paroxysmal tachycardia. They occur mainly at night. The most dangerous consequence The development of the disease is considered to be atrial fibrillation. This deviation can lead to the death of the patient.
A thorough history will also be required to make a diagnosis. This is due to the hereditary nature of Brugada syndrome. In patients with a history of sudden death in their family, doctors should give Special attention work of the heart. Confirmation of the presence of pathology also involves conducting genetic tests that can identify mutations in DNA sections. To assess the structure of the heart, ultrasound is used, which makes it possible to take specific photos of the organ. Measurements are taken from the images, and contractile function is also assessed.

Treatment
The fight against defeat is much more difficult. This is due to the lack of adequate and timely diagnosis pathology. In this case, patients can be treated both with the help of medications and with the use of surgical techniques that involve the installation of a pacemaker. At the same time, conservative methods are significantly inferior to radical ones in effectiveness.
Drug therapy
Not all antiarrhythmic drugs can be used in patients with a genetic abnormality. This is due to the different mechanism of action of these medications. For example, treatment with sodium channel blockers for Brugada syndrome can lead to a worsening of the patient's condition. For this pathology, drugs such as Quinidine and Disopyramide are used. They demonstrate good results in the fight against attacks of paroxysmal tachycardia. At the same time, the answer to drug treatment observed in only 60% of patients.
Defibrillator installation
Implantation of the device is currently considered the most effective way to treat Brugada syndrome. It is necessary when clinical signs disease, detection of fibrillation during Holter monitoring, as well as positive test using sodium channel blockers. A cardioverter-defibrillator helps prevent sudden death of a patient by correcting the heart rhythm.
The outcome of the pathology is determined by the intensity of its clinical manifestations. If the patient only has specific signs on the electrocardiogram, the prognosis is favorable, especially if timely treatment. Without a defibrillator, there is a high risk of sudden cardiac arrest.
There are studies that indicate the multifactorial nature of the disease. Doctors are inclined to believe that the intensity of clinical signs of damage is influenced not only by the type of genetic mutation that led to the problem, but also by the environmental situation, as well as the hormonal background in the human body and his lifestyle.
Phenotypic manifestations are used to predict disease outcome and response to treatment. It has been proven that those at risk for fatal complications of Brugada syndrome are patients with constantly recurring fainting, agonal breathing against the background of paroxysmal tachycardia at night, as well as with convulsions of unknown etiology. For such patients, doctors recommend installation of an implantable cardioverter-defibrillator, which reduces the chance of sudden death.
At the same time, there is still debate about the justification of using the device in patients who do not encounter clinical manifestations of Brugada syndrome in everyday life.
A number of doctors are inclined to believe that if there is a specific pattern on the ECG, patients require surgery. Others argue that implantation is justified only when symptoms of the lesion appear.
Prevention of the development of Brugada syndrome has not been developed. Prevention of the problem comes down to karyotyping of parents at the stage of pregnancy planning. To prevent the formation of fatal complications, it is important to diagnose the existing problem in a timely manner.
NEUROLOGIST'S HANDBOOK

Why cardiac pathology in the pathology blog nervous system?! Because loss of consciousness is one of common reasons referrals (appeals) to a neurologist.
In modern clinical medicine a special number of diseases and syndromes associated with high risk sudden death in at a young age. These include sudden infant death syndrome, long QT syndrome, sudden unexplained death syndrome, arrhythmogenic right ventricular dysplasia, idiopathic ventricular fibrillation, etc. The most “mysterious” disease on this list is Brugada syndrome(SB) and it is he who, according to many experts, is “responsible” for more than 50% of sudden non-coronary deaths at a young age (i.e. in individuals with no organic changes in the coronary arteries and myocardium).
Brugada syndrome- clinical electrocardiographic syndrome, which combines [ 1 ] frequent cases [due to the development of polymorphic ventricular tachycardia (VT) or ventricular fibrillation (VF)] syncope or sudden cardiac death (SCD) and [ 2 ] the presence on the electrocardiogram (ECG) of a special form of right bundle branch block (RBBB) with ST segment elevation (in the right precordial leads), as well as [ 3 ] no organic changes coronary arteries and myocardium (SB is a family, genetically inherited syndrome related to channelopathies [see below] and included in the concept of “primary electrical heart disease”).
read also the article “Diagnostics of ventricular arrhythmias” by A.V. Strutynsky, A.P. Baranov, A.G. Elderberry; Department of Propaedeutics of Internal Diseases, Faculty of Medicine, Russian State Medical University (magazine “General Medicine” No. 4, 2005) [read]
Data on the prevalence of SB are conflicting. The incidence of SB is lower in Western countries (1 - 2 cases per 10,000 people) and increases in Southeast Asia (more than 5 per 10,000 [provoking factors for the development of SB in this region can be considered the content of a large amount of potassium in food and a hot climate ; research on this issue continues]). According to a number of authors, the indicated incidence rates of SB are far from reality due to insufficient diagnosis of this disease.
In accordance with ECG changes, three types of ST segment elevation in SB are distinguished. In type I, there is a pronounced rise in the J point, arching of the ST segment and T-wave inversion in leads V1 and V2. In type II, a saddle-shaped rise in the ST segment (more than 1 mm) is recorded. In type III, ST segment elevation is less than 1 mm. In accordance with the consensus document, type I ECG changes are indicative of the diagnosis of SB (in essence, there are two morphologies of the ECG pattern of SB: the first is “coved type” [“dome-shaped”] and the second is “saddle-back” [“saddle-shaped” ], including the second and third types described earlier).

read also the article “ Diagnostic value J-waves” Limankina I.N., St. Petersburg Psychiatric Hospital No. 1 named after. P.P. Kashchenko, Russia (magazine “Emergency Medicine” No. 1, 2013) [read]
Pay attention e! Diagnosis of SB presents significant difficulties for doctors, as evidenced by the frequency diagnostic errors. One of the reasons for this is the doctors’ insufficient knowledge of the clinic and diagnostic criteria of this disease (differential diagnosis of the ECG pattern of SB from ECG changes in other conditions can be quite difficult even for an experienced cardiologist). The ECG pattern of SB can be hidden, dynamic, and appear against the background [ 1 ] fever, [ 2 ] intoxication, [ 3 ] vagal stimulation, [ 4 ] electrolyte changes, [ 5 ] taking certain medications (see table below).
note! The peculiarity of SB is that typical ECG pattern, unlike long QT syndrome and other congenital arrhythmogenic channelopathies, is NOT permanent. Characteristic ECG changes are more pronounced in the period before the development of VT (or VF) or immediately after it, and can be provoked by a number of medications.

It has been established that SB is a hereditary (i.e. genetically determined) disease caused by a mutation of the SCN5A gene, located in the short arm of the 3rd chromosome (3p21-24), encoding the biosynthesis of protein subunits of α-sodium channels of cardiomyocytes, i.e. SB is an example of a channelopathy (the Na channel is a complex membrane protein that regulates the rapid flow of sodium depending on the phase of the transmembrane action potential; in SB, due to the SCN5A mutation, in the epicardial cells of the right ventricle there is a decrease in the number of Na channels and/or their accelerated inactivation - this is manifested by a decrease in INa density and the occurrence of premature repolarization of the epicardium). The disease has an autosomal dominant pattern of inheritance. Today, about five genes are known to be responsible for the development of this disease; a mutation in any of them can lead to the development of the disease (more than 80 mutations responsible for the development of SB have been described). However, it should be remembered that in approximately 15% of cases, patients with BS do not have a characteristic family history, which may be a consequence of sporadic mutations.
As noted above, SB is the cause of more than 50% of sudden non-coronary deaths in young people. Clinical manifestations of the syndrome usually develop at the age of 30 - 40 [-50] years (but the disease can manifest itself at any age, both at an older age and up to the neonatal period). Men get sick 8 to 10 times more often than women. The clinical picture of the disease is characterized by the frequent occurrence of syncope against the background of attacks of VT (or VF) and SCD, mainly during sleep, as well as the absence of signs of organic myocardial damage at autopsy. In the vast majority of cases, attacks of VT in SB occur in the evening and at night (from 18 to 06 hours), more often in the second half of the night, which confirms the role of increased vagal influences in the occurrence of VF in SB (this circadian pattern also indicates differences in the pathogenesis of the occurrence fatal arrhythmias in patients with SB and coronary heart disease, when the main circadian peak of SCD occurs in the early morning hours).
Remember! It is necessary to exclude SB in the following cases: [ 1 ] the appearance of characteristic changes on the ECG (see above); [ 2 ] syncope (fainting) unknown origin; [3 ] episodes of polymorphic ventricular tachycardia; [ 4 ] cases of sudden death in the family, especially in practically healthy men aged 30 - 50 years.
reference Information. Clinical features cardiogenic fainting (syncope) associated with heart rhythm disturbances are their suddenness, connection with physical activity and emotional factors associated symptoms autonomic dysfunction. When analyzing the stages of development of fainting, attention is paid to a short presyncope period with unpleasant sensations or pain in the heart area, sensations of “stopping”, “fading” of the heart or palpitations, unsystematic dizziness, severe general weakness, darkening before the eyes, ringing in the ears, a feeling of heat in the head, unpleasant sensations in the epigastric region. In some cases, the presyncope period in the clinic of cardiogenic syncope may be completely absent. In such a situation, fainting manifests itself as a sudden fall and resembles an epileptic paroxysm (including, perhaps [especially in the case of prolonged general cerebral hypoperfusion] convulsive syndrome and/or involuntary urination, and/or tongue bite). At the same time, patients have pronounced autonomic disorders - pallor of the skin, coldness, hyperhidrosis, frequent shallow breathing with difficulty exhaling, bradycardia up to 32 - 48 beats per minute, decreased blood pressure to 90/60 mm Hg. As a rule, loss of consciousness during arrhythmogenic syncope is short-lived, up to 3 minutes. When differentiating from epilepsy, the fact that after syncope there is complete and fast recovery consciousness without amnesia, increased drowsiness. The post-syncope period in cardiogenic syncope is often present, its duration varies from 5 minutes to an hour, there is general weakness, malaise, sometimes headache, and discomfort in the heart area. Distinctive feature, the so-called arrhythmogenic syncope, are their stereotypicality, tendency to seriality and close connection with the factor of lack of stabilization of the heart rate (source: article “Cardiogenic syncope through the eyes of a neurologist” T.V. Mironenko, L.N. Ivanova, O.N. Chmelyuk; Lugansk State Medical University (Journal of Neurology named after B.M. Mankovsky, No. 2, 2013) [read]).
read also the post: Transient loss of consciousness: fainting or fainting-like state?!(at laesus-de-liro.livejournal.com)
Most scientists believe that the severity of clinical manifestations of SB is determined by the degree of damage to sodium channels: if less than 25% of ion channels are damaged ECG changes ST segment and rhythm disturbances are induced only pharmacologically - by the introduction of sodium channel blockers, and with an increase in the number of damaged sodium channels (more than 25%), the likelihood of manifesting an ECG pattern and the risk of SCD sharply increases. Approximately 80% of patients who experienced clinical death had a history of syncope, including convulsions, before this dramatic episode. In a number of patients, attacks can occur without loss of consciousness, in the form of severe general weakness and interruptions in the functioning of the heart, with so-called presyncope states (lipotymia). There are descriptions in the literature of an asymptomatic variant of this SB with a mutation associated with a disruption of the connection between calmodulin and the sodium channel.
note! To date, researchers have described various rhythm disturbances that occur in SB: supraventricular arrhythmias, atrial fibrillation (AF), atrioventricular nodal tachycardia, atrioventricular block, sick sinus syndrome, but the most common and life-threatening are paroxysms of polymorphic ventricular paroxysmal tachycardia of the “pirouette” type ( torsades de pointes) and VF.
When ECG criteria are met, the diagnosis of SB is made in patients [ 1 ] with documented ventricular tachyarrhythmia (polymorphic VT or VF, induction of ventricular tachycardia with programmed electrical stimulation), or [ 2 ] with a burdened family history (SCD in relatives under 45 years of age, ECG pattern of type 1 SB in relatives), or [ 3 ] in the presence of symptom-related arrhythmia (syncope, attacks of nocturnal respiratory arrest).
The following clinical and electrocardiographic forms and variants of SB are distinguished: [ 1 ] full form (typical ECG picture with syncope, presyncope, cases of clinical death or SCD due to polymorphic VT); [ 2 ] typical ECG picture in asymptomatic patients without a family history of SCD or SB; [ 3 ] typical ECG picture in asymptomatic patients, family members of patients with full form SB; [ 4 ] typical ECG picture after pharmacological tests in asymptomatic subjects, family members of patients with the full form of SB; [ 5 ] typical ECG picture after pharmacological tests in patients with repeated syncope or idiopathic AF; [ 6 ] typical ECG picture with obvious RBBB, ST segment elevation and prolongation P-R interval; [7 ] typical ECG picture with ST segment elevation, but without prolongation of the P-R interval and RBBB; [ 8 ] incomplete RBBB with moderate ST segment elevation; [ 9 ] isolated prolongation of the P-R interval.
To verify SB, it is possible to conduct drug stress tests using class IA antiarrhythmic drugs, such as ajmaline, flecainide, procainamide, pelsicainide. Such a test should be carried out if SB is suspected in persons with frequent occurrence of syncope in intensive care conditions under constant ECG monitoring, which positive result Brugada-like changes are recorded.
Differentiate SB necessary with a phenocopy (or phenotype) of Brugada (FB), which occurs transiently against the background of metabolic disorders, electrical trauma, ischemia, taking certain medications, and for other reasons. Although this concept requires confirmation, it is believed that in FB the pharmacological test will be negative, and genetic testing will not confirm the presence of mutations responsible for the development of SB (for direct DNA diagnosis of SB, analysis of the coding sequence of the genes SCN5A, KCNQ1, KCNE1, KCNH2, KCNE2 and etc.).

Remember! It is necessary to carry out differential diagnosis SB and a number of diseases that can cause similar ECG manifestations: arrhythmogenic dysplasia of the right ventricle (RV), myocarditis, cardiomyopathy, Chagas disease (myocarditis), Steinert disease, mediastinal tumor. Pathologies that cause ST segment elevation in the right precordial leads include: acute myocarditis, acute pericarditis, hemopericardium, right ventricular infarction, aortic aneurysm dissection, acute thromboembolism pulmonary artery, central nervous system anomalies, Duchenne muscular dystrophy, Friedreich's ataxia, thiamine deficiency, hypercalcemia, hyperkalemia, mediastinal tumor compresing right ventricular outflow tract, arrhythmogenic right ventricular cardiomyopathy, long QT interval Type 3, right bundle branch block, left bundle branch block, left ventricular hypertrophy, early repolarization syndrome, hypothermia.
Drug approaches to the treatment of SB are being actively developed, but the results of these studies still remain controversial. There is currently virtually no convincing, consistent data on the effectiveness of any medications in the long-term prevention of VT/VF attacks. For patients at high risk of SCD, a treatment option that increases life expectancy is implantation of a cardioverter-defibrillator.
Thus SB is a genetically determined disease with cardiac arrhythmia, which is characterized by frequent occurrence of syncope against the background of episodes of polymorphic VT and VF. Often the outcome of the disease is SCD. In all patients with syncope of unknown etiology, nocturnal paroxysms of suffocation, cases of SCD in the family (especially at a young age and at night), a typical ECG pattern, it is necessary to exclude SB. To do this, such patients should undergo pharmacological tests, dynamic ECG examination (including the patient’s relatives), and Holter monitoring. One of the most reliable methods for diagnosing SB is molecular genetic research. To date, the only method for the treatment of VT/VF, as well as the prevention of SCD in SB, is the implantation of an automatic cardioverter-defibrillator.
Read more about SB in the following sources:
article (lecture) “Brugada Syndrome” by O.L. Bockeria, A.V. Sergeev; FSBI " Science Center cardiovascular surgery them. A.N. Bakulev", Moscow (magazine "Annals of Arrhythmology" No. 1, 2015) [read];
article “Brugada syndrome: cellular mechanisms and approaches to treatment” by L.A. Boqueria, O.L. Bockeria, L.N. Kirtbaya Scientific Center for Cardiovascular Surgery named after. A.N. Bakulev RAMS, Moscow (journal “Annals of Arrhythmology” No. 3, 2010) [read];
article “Electrophysiological basis of therapy for Brugada syndrome” Maltseva A.S., Strogonova V.V.; FSBEI HE First Moscow State Medical University named after. THEM. Sechenov Ministry of Health of the Russian Federation, Moscow (journal of scientific articles “Health and Education in the 21st Century” No. 3, 2017) [read];
article “Brugada syndrome: from primary electrical heart disease to morphological substrate” by T.A. Pavlenko, O.V. Blagova; First Moscow State Medical University named after. THEM. Sechenov, 6th year, Faculty of Medicine, Moscow; First Moscow State Medical University named after. THEM. Sechenov, Department of Faculty Therapy No. 1, Moscow (magazine "Archive internal medicine» No. 2, 2016) [read];
article “Brugada Syndrome” by S.D. Mayanskaya, N.A. Tsibulkin; Kazan State Medical University; Kazan State medical Academy, Kazan (magazine " Practical medicine» No. 3, 2015) [read];
article “Brugada syndrome: literature review and clinical observation” by T.G. Vaikhanskaya, T.T. Gevorkyan, T.V. Krushevskaya, I.B. Ustinova, T.V. Kurushko, V.F. Tops, O.L. Polonetsky, L.I.; Plashchinskaya Republican Scientific and Practical Center “Cardiology”, Minsk (magazine “Medical Business” No. 6, 2013) [read];
article “Brugada Syndrome” Mangusheva M.M., Aliakberova G.I., Valeeva A.R., Teregulov Yu.E.; Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan, Kazan State Medical University (magazine “Practical Medicine” No. 7, 2011) [read];
article “The appearance of electrocardiographic signs of Brugada syndrome during therapy with the class IC antiarrhythmic drug etacizine. Description of the case" L.M. Makarov, V.N. Komolyatova; Center for Syncope and Cardiac Arrhythmias in Children and Adolescents of the FMBA of Russia, Children's Clinical Hospital No. 38 of the Center for Epidemiology and Epidemiology of the FMBA of Russia; KB No. 85 FMBA of Russia Department of Clinical Physiology and Functional Diagnostics IPK FMBA of Russia (magazine “Cardiology” No. 4, 2011) [read]
© Laesus De Liro